CytExpert Tools for 21 CFR Part 11 Compliance
All computer systems that store data used to make Quality decisions or data that will be reported to the FDA must be compliant with 21 CFR Part 11. The purpose of the law is to define the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records.
21 CFR Part 11 refers to the section in the Code of Federal Regulations (CFR) that sets forth the United States Food and Drug Administration’s (FDA) guidelines on using electronic records and electronic signatures. The regulation applies across a range of regulated industries including pharmaceuticals, biologics, medical devices and food products (human and veterinary). Companies in these segments doing business in the U.S., providers of raw materials and components to pharmaceutical companies and contract labs commissioned to perform analysis must all operate to ensure electronic records and electronic signatures are trustworthy and reliable.
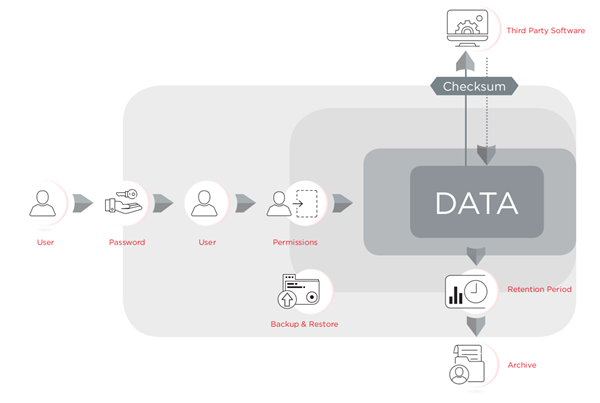
The regulation requires the organization to have three levels of control in place: administrative controls such as policies for electronic records, procedural controls such as SOPs for using the system, and technical controls for the functions built into software that ensure the reliability and integrity of electronic records and signatures. Software can be designed to facilitate compliance with 21 CFR Part 11 technical controls, however the laboratory personnel are responsible for providing policies and procedures and to ensuring their proper use and ensure their process is compliant with the regulations. The Electronic Record Management tools included in CytExpert v2.3 or higher, are available for the CytoFLEX flow cytometer platform, have been designed to facilitate compliance with 21 CFR Part 11. CytExpert for CytoFLEX is a password-protected software that controls the instrument's operation, safeguards data integrity control using checksum and audit trails, and provides document control using signatures, signature hierarchy and archiving.