Viral RNA Extraction
Reagent Kits: RNAdvance Viral or RNAdvance Viral XP
Extract high-quality RNA from Saliva or Swab Transport Media samples using SPRI paramagnetic bead-based technology. Our viral RNA extraction kits are available for your unique input sample types and collection devices. RNAdvance Viral XP offers higher throughput capabilities and the RNAdvance Viral kit is optimal for extracting from a variety of challenging sample inputs.
- Produces high-integrity viral RNA for use with PCR-based applications for infectious disease and pathogen research
- Performance Limit of Detection (LoD) demonstrated at 1 copy/uL for viral RNA
- Automation-ready, viral extraction quickly adapted to liquid handling platforms
For research use, not intended for diagnostic purposes.
Explore Viral Models
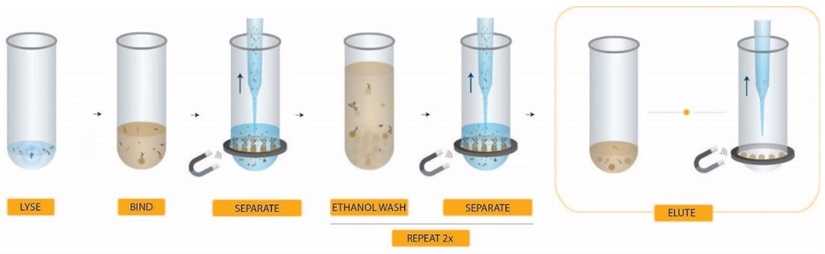
RNAdvance Viral Workflow Steps
RNAdvance Viral Product Features
RNAdvance Viral XP isolates RNA from Universal Transport Media. RNAdvance Viral isolates RNA from Saliva and Swab Transport Media.
The RNAdvance Viral XP and RNAdvance Viral isolation kits purify intact RNA suitable for downstream PCR-based applications. Limit of Detection (LoD) of 1 copy/µL for viral RNA.
Combining RNAdvance Viral chemistry with automation offers a high-throughput processing solution requiring minimal hands-on-time.
RNAdvance Viral Product Specifications
| Application Uses | Research Use, RNA Isolation, RNA Extraction, Nucleic Acid Sample Prep, PCR |
| Format | Liquid |
| Starting Sample Material | Saliva and Swab in Universal Transport Media |
| Automated Available | Yes |
| Item Specifications Referenced | C63510 |





