QA/QC Products
These products are frequently used in quality assurance and quality control workflows for biologics manufacturing.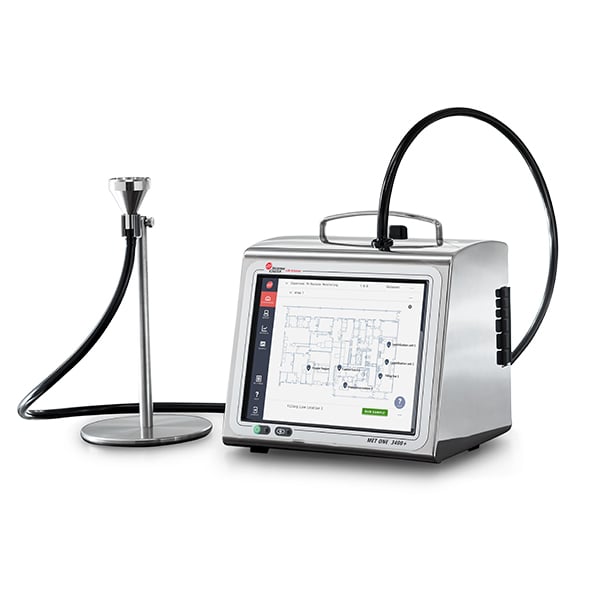
MET ONE 3400+
Use the MET ONE 3400+ portable air particle counter for environmental monitoring in cleanrooms
HIAC 9703+
Use the HIAC 9703+ particle counter to count particulates for final product quality testing for injectables/ parenterals.

Facility Monitoring Systems (FMS)
Flexible and scalable particle monitoring system for GMP compliant pharmaceutical manufacturing leveraging MET ONE instruments.

Optima AUC
Use the Optima AUC analytical ultracentrifuge for in-solution formulation characterization, stability and lot release testing.
MET ONE HHPC Plus
Use the MET ONE HHPC+ handheld particle counters for measuring particulates in cleanrooms

ANATEL PAT700
Use the ANATEL PAT700 analyzer for fast on-line release testing for conductivity, TOC analysis and final rinse in validated CIP programs.

LS 13 320 XR
Use the LS 13 320 XR analyzer to measure particles for final formulation during drug stability formulation testing.
QbD1200
Use the QbD1200 analyzer for point-of-use grab sample analysis for TOC Analysis or during CIP program development, validation, and/or for final rinse grab sample analysis for validated CIP programs.Products and methods described are not intended for use in diagnostic procedures.

