Viral Vector Characterization
Cell and gene therapy involves leveraging a gene delivery vehicle to introduce a therapeutic into the body. Many gene therapy companies are working with Adeno-Associated Virus (AAV) as their gene delivery vehicle of choice. AAVs are ideal because the viral capsids can be modified to carry a therapeutic cargo. Researchers first need to determine what percent of their viral capsids are intact, they then need to understand how many intact viral capsids are full. This transgenic cargo is critical for the success of the therapy.
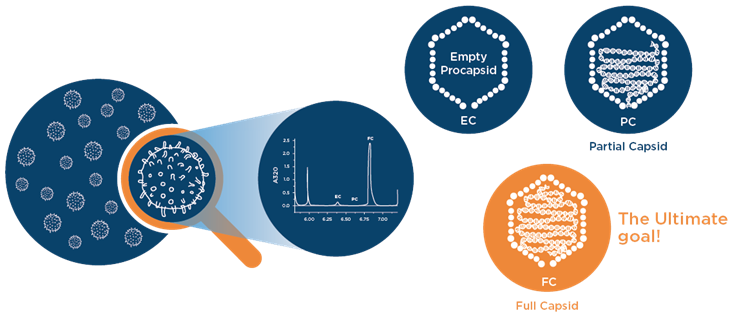
It’s particularly important for researchers to understand the viral load of these particles because viral particles that do not contain the full target therapeutic gene are unlikely to produce therapeutic activity, instead these partial capsids might produce adverse reactions, such as an immunogenic response. Researchers need to understand the quality and purity of a given viral prep, and many are turning to analytical centrifugation for this insight.
The solution to measuring rAAV vector homogeneity, purity (and more): analyze viral particles in solution.Recombinant adeno-associated viral vectors (rAAV) could hold great promise for new, life-saving gene therapies.
But while classic techniques such as dynamic light scattering (DLS) or high performance liquid chromatography (HPLC) can characterize rAAV heterogeneity and aggregation, they simply don’t provide sufficient resolution for quantifying homogeneity and viral particle load. When it comes to producing clinical-grade rAAV vector preparations, one obstacle has always been achieving high-resolution measurements.
As gene therapy researchers know, with so much at stake in early product development, failing fast is fundamental. Adoption of analytical ultracentrifugation makes it possible to produce clinical-grade rAAV vectors, unlike the other usual technologies.
The main obstacle? Lack of a high-resolution, quantitative technique for monitoring therapeutic quality with regard to:
- Homogeneity
- Purity
- Product consistency
- Viral fullness
The ideal solution: in-solution analysis with analytical ultracentrifugation (AUC).
As scientists such as those at Genethon have discovered, by enabling matrix-free analysis of rAAV vector preparations—independent of serotype and transgene—AUC helps them:
- Determine viral assembly state or homogeneity
- Empirically quantify aggregation
- Determine subparticle contamination
- Quantify mass to accurately measure genetic payload
View this webinar to learn how Genethon’s Christine LE BEC and her team have used AUC to successfully characterize scAAV and ssAAV vectors—including homogeneity and viral particle load.
Product for Characterizing Viral Vectors

Optima AUC
The next generation analytical ultracentrifuge frequently used in viral vector QC to quantify the percent load in AAV capsids

