Biomek i-Series Automation of the Beckman Coulter GenFind V3 Blood and Serum DNA Isolation Kit
Biomek Automated Genomic Sample Prep Accelerates ResearchIntroduction
The GenFind V3 Blood and Serum DNA Isolation Kit from Beckman Coulter isolates high quality genomic DNA from fresh or frozen whole blood and serum containing Citrate, EDTA, or Heparin anticoagulants, as well as from cultured cells. The kit produces a high recovery of DNA for downstream applications such as agarose gel analysis, restriction enzyme digestion, PCR amplification, and next-generation sequencing library construction. GenFind V3 uses an improved cell lysis buffer and Proteinase K treatment to rupture cell membranes and digest proteins. DNA is then immobilized using Beckman Coutler’s patented SPRI (Solid Phase Reverse Immobilization) paramagnetic bead technology by adding a magnetic bead reagent. This differential binding of DNA enables separating DNA with the magnetic field, followed by rinsing away contaminants with multiple simple wash procedures, leaving the genomic DNA ready for elution. SPRI also enables fast separation, easy manipulation and simple automation compared to traditional vacuum filtration and centrifugation technologies. In this technical note, we demonstrate the automated performance of the Beckman Coulter GenFind V3 kit on the Biomek i7 Hybrid Genomics Workstation.
When compared to manual operations, the Beckman Coulter GenFind V3 kit automated on Biomek platforms provide
- Reduced hands-on time and increased throughput
- Option to run the method end-to-end with only setup and tear-down touch points
- Reduction in pipetting errors
- Standardized workflow for improved results
- Quick implementation with demonstrated methods
- Knowledgeable support for reagents, automation and methods all from a single vendor
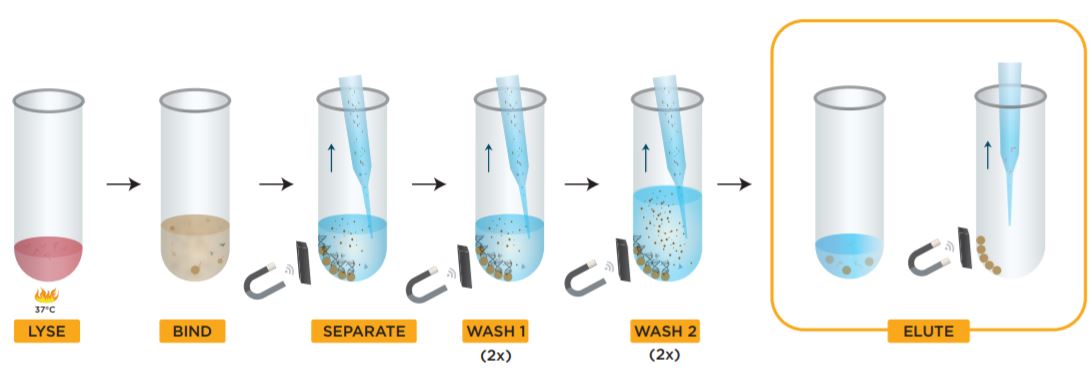
Figure 2. Beckman Coulter GenFind V3 kit protocol
Automated Method
We automated the Beckman Coulter GenFind V3 kit protocol on the Biomek i7 Hybrid, incorporating on-deck lysis. In general, the automated protocol enables DNA extraction from 1-96 blood and serum samples up to 400 µL, in approximately 4 hours (Table 1).
The handling of blood and blood waste require special precautions and waste disposal procedures. Therefore, the automated GenFind V3 method isolates blood waste into waste plates, facilitating proper disposal of biohazardous waste.
| Major Process Description | Automated/ Hands on Time | |
| 24 Samples | 96 Samples | |
| Sample Extraction - 400 µL input | ||
| Prepare Reagents/ Set up Inst | 30 min | 30 min |
| Method Run Time | 2 hr, 29 min | 3 hr, 24 min |
| Total | 2 hr, 59 min | 3 hr, 54 min |
| Major Process Description | Automated/ Hands on Time | |
| 24 Samples | 96 Samples | |
| Sample Extraction - 200 µL input | ||
| Prepare Reagents/ Set up Inst | 30 min | 30 min |
| Method Run Time | 2 hr, 8 min | 2 hr, 51 min |
| Total | 2 hr, 38 min | 3 hr, 21 min |
* Timing does not include reagent and sample thawing, blood/serum collection, homogenization or dissection.
Table 1. Estimated run times for Beckman Coulter GenFind V3 automated method on the Biomek i7 Hybrid.
Demonstrated Method Interface (DMI)
Three modules used to set up the GenFind V3 protocol provide the user with full instructions to set up the method and reduce set up errors.
1. Biomek Method Launcher (BML)
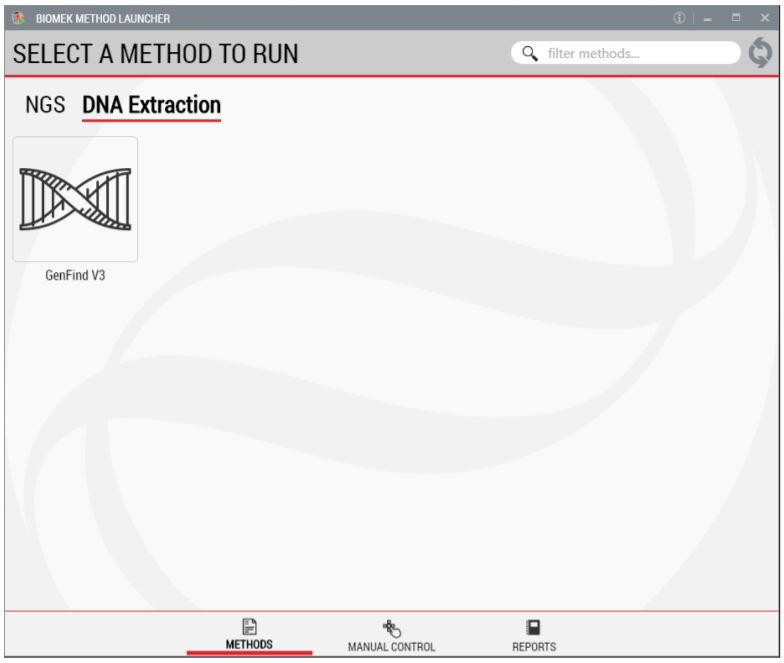
BML is a secure interface for selecting methods without affecting method integrity and manual control (Figure 3).
Figure 3. Biomek Method Launcher provides an easy interface to start the method
2. Method Options Selector (MOS)
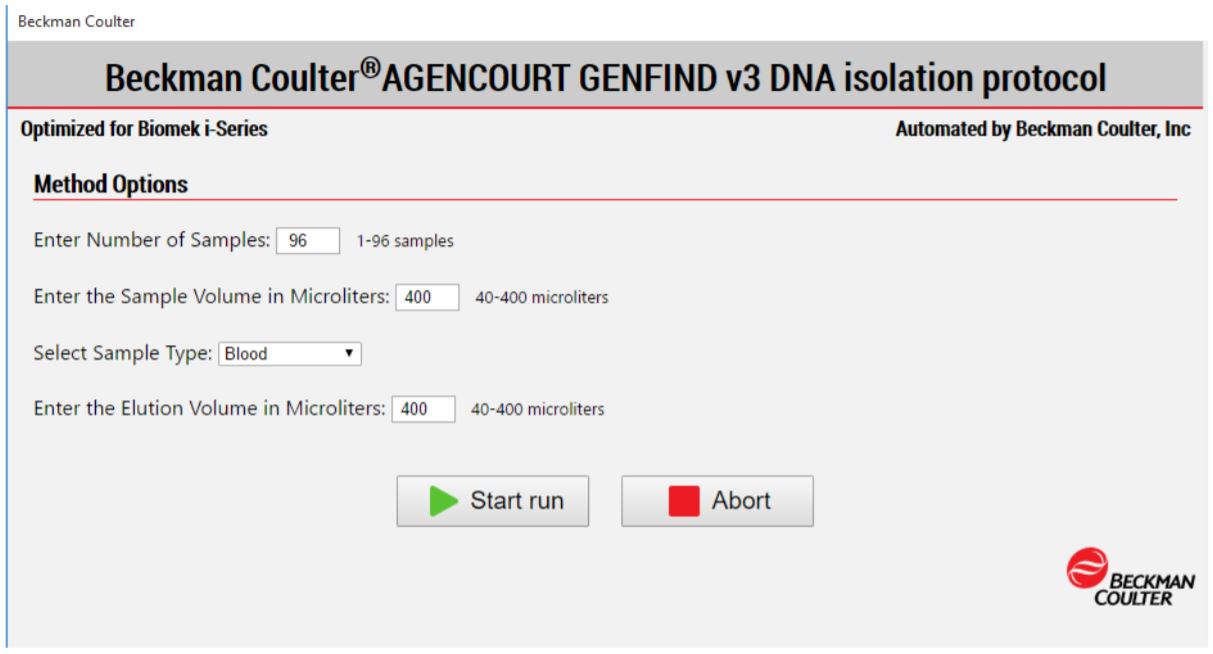
MOS enables the selection of run-time options to maximize the flexibility in the set up of the method execution (Figure 4). For the GenFind V3 method, the automated protocol allows for 1-96 samples in the volume range of 40-400 µL for processing.
Figure 4. Biomek Method Options Selector indicates sample number and processing options
3. Guided Labware Setup (GLS)
GLS is generated based on user selected options in the MOS and provides the user specific text and step-by-step graphical setup instructions with reagent calculations (Figure 5).
Figure 5. Guided Labware Setup indicates reagent volumes and guides the user for correct deck setup
Experimental Design
Whole blood was collected from four individual donors in blood collection tubes coated with EDTA, Citrate, and Heparin anticoagulants. Four technical replicates for each donor were used for a total of 48 samples of 400 µL aliquots (16 EDTA, 16 Citrate, 16 Heparin). Genomic DNA was then extracted using the GenFind V3 Blood and Serum DNA Isolation automated method implemented on the Biomek i7 Hybrid platform. All extracted genomic DNA samples were analyzed using a NanoDrop™ 8000 (Thermo Fisher Scientific). 14 samples (four EDTA, five Citrate, and five Heparin) were randomly selected and analyzed using 2200 TapeStation using the Genomic DNA ScreenTape kit (Agilent Technologies). Those same 14 genomic DNA samples were then converted to NGS sequencing libraries using the Kapa HyperPlus Kit implemented on the Biomek i5 NGS Workstation. NGS library QC was performed using the 2100 Bioanalyzer with the High Sensitivity DNA kit (Agilent Technologies).
Results
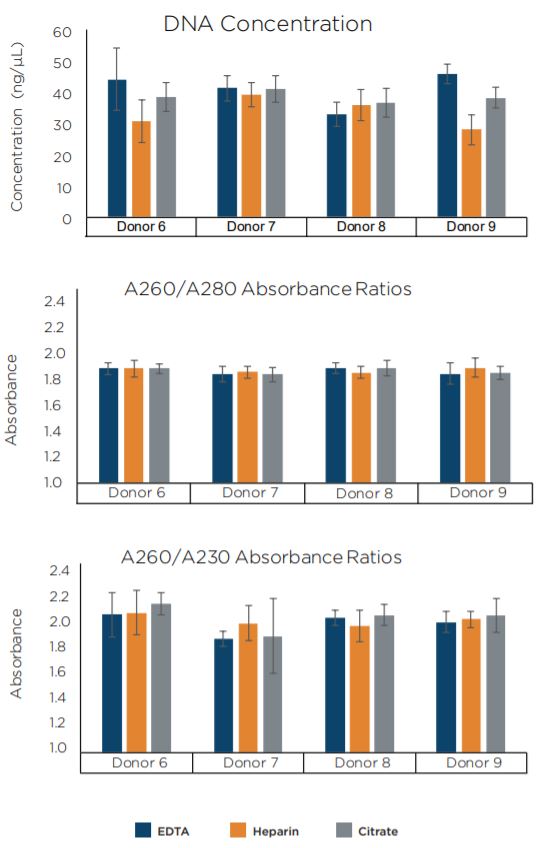
The quantity as well as quality of DNA extracted from the automated protocol were comparable to those of the manual protocol (Figure 6). The DNA Integrity Number (DIN) for 14 selected samples were also calculated by TapeStation to further estimate DNA quality. Both methods indicate the extraction of high quality DNA, as indicated by their high DIN scores (Table 2).
To demonstrate the use of the extracted DNA in downstream applications, we constructed NGS libraries from the same 14 selected samples using the Kapa HyperPlus Kit. Successful library preparation was verified using a Bioanalyzer. Certain anticoagulants such as Heparin act as PCR inhibitors. The resulting traces indicate successful library construction and that the inhibitors were successfully removed through the GenFind V3 protocol (Figure 7).
Figure 6. Average DNA concentration and purity ratios of replicate samples and blood tube types as indicated by NanoDrop 8000
| Donor | Blood Tube Type | DIN |
| D6 | EDTA | 9.6 |
| D6 | Heparin 9 | 9.5 |
| D6 | Citrate | 9.6 |
| D7 | EDTA | 9.2 |
| D7 | Heparin | 9.4 |
| D7 | Heparin | 9.6 |
| D7 | Citrate | 9.5 |
| D8 | EDTA | 9.1 |
| D8 | Heparin | 9.6 |
| D8 | Citrate | 9.5 |
| D8 | Citrate | 9.2 |
| D9 | EDTA | 9.5 |
| D9 | Heparin | 9.6 |
| D9 | Citrate | 9.2 |
Table 2. DIN scores for randomly selected samples analyzed on Agilent TapeStation 2200