Cydem VT System: A Comparison to Traditional Clone Screening Platforms

Introduction
The Cydem VT Automated Clone Screening System is a fully automated solution which allows for the screening of up to 96 CHO clones in parallel to support cell line development (CLD) workflows. The system combines several trusted Beckman Coulter Life Sciences technologies into a new and innovative integrated solution and provides the data required to make crucial decisions with confidence during cell line development. The system provides individual gassing control with CO2, O2, and N2 for all cultivations, with daily base addition (as required, based on online pH measurements) and feeding events and determination of cell concentration, viability and IgG concentration.
In this application note, the results of a clone screening experiment performed with the Cydem VT system using 46 different clones cultured in duplicates are shown. Additionally, these 46 clones were simultaneously cultured in a traditional deep well fed-batch (DWFB) culture and in a 15 mL microbioreactor system.
Methods
Since a prototype version was used, the shown methods for standard curve generation and cell seeding method deviate slightly from those for the final product. Refer to the Instructions for Use (IFU) manual for information on the current method.
Before running the clone screening experiment, a standard curve with the targeted antibody was generated for online titer determination. Further, the system was decontaminated and prepared for experiment start (see Instructions for Use (IFU) manual for detailed instruction). The selected 46 clones were grown in a shaken incubator, and the 24-well cell source plates were prepared with a known viable cell density (VCD) of approximately 2*106 cells/mL. The cells were seeded with the known VCD seeding method at a target cell density of 0.4*106 cells/mL at 5 mL. Cells were seeded in duplicates in the four Cydem VT system microtiter plates. The dissolved oxygen (DO) setpoint was set at 50% and the following pH setpoints were selected: CO2: 7.1, base trigger: 6.9, base target: 7.0. For liquid base additions 0.5 M NaOH was added, with a pH control scale factor of 190. The bioreactor was shaken at 800 rpm, 36.5°C.
Feeding events were scheduled daily, starting on day 3, with both a feed solution and additional glucose feeding (no premixing) based on previously determined feed schedules. Determination of cell concentration and viability was scheduled every day, and titer measurements were run from day 8 onwards. On days 7, 10 and 14, an output plate was generated for offline titer measurements on a Cedex Bio HT analyzer and checked for correlation.
In parallel, clone screening experiments using the same 46 clones were started in DWFB as well as in the 15 mL microbioreactor system. As in the Cydem VT system, both experiments started with a seeding density of 0.4*106 cells/mL.
For the DWFB, feed solution and glucose were applied every other day except the day before the weekend where a higher amount of feed and glucose solution was added. On days 7, 10, and 14, offline values for cell count and titer were determined with a Cedex Bio HT analyzer.
For the experiment in the 15 mL microbioreactor system, feed solution was added from day 1 onwards. Glucose solution was added based on glucose determination on days 3, 5, 7, 10, 12 and 14 of the culture. On the same days, samples for cell count and titer determination were analyzed on a Cedex Bio HT analyzer.
For pH regulation, 6% (w/v) of Na2CO3 was used. Although, feeding schedules were slightly different on the individual days, the total relative feeding volume between instruments differs less than 5%. Variation in feeding is due to already established feeding strategies based on offline glucose measurements.
Results
In the Cydem VT system, pH values were continuously measured in every well and monitored over the cultivation time of 14 days (Figure 1). Each well represents a different clone. For every clone a different pH profile is visible. This can be attributed to the different growing behaviors of each clone. Every well was individually regulated to the pH setpoint.
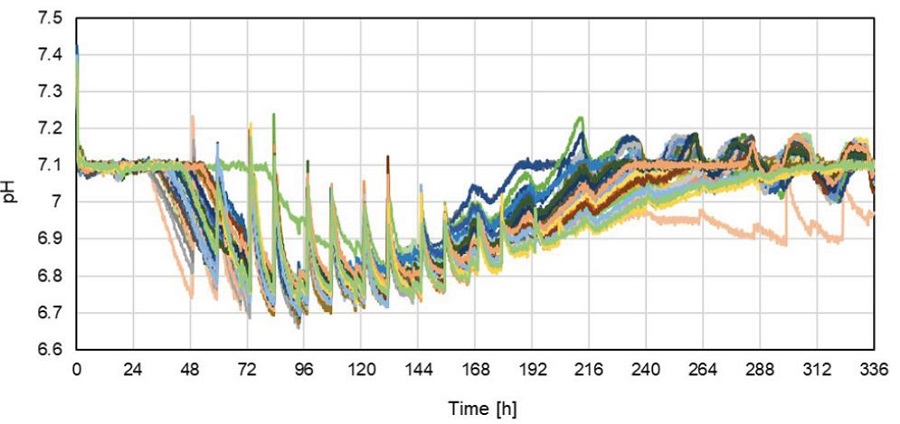
Figure 1. pH profile of 46 individual wells. Each line represents a single well/clone.
DO profiles measured in 46 individual wells were continuously detected over the cultivation time of 14 days in the Cydem VT system (Figure 2). Each well represents a different clone. DO levels for every well were kept around the setpoint of 50% over the duration of the experiment.
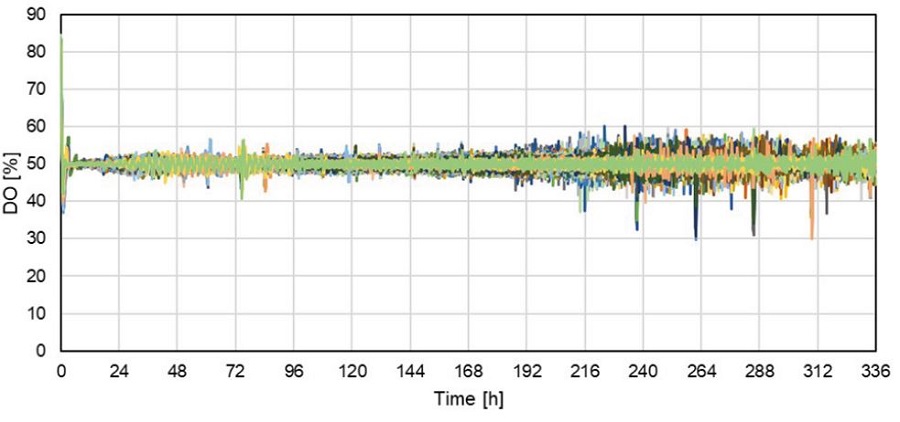
Figure 2. DO profiles of 46 individual wells. Each line represents a single well/clone.
Another feature of the Cydem VT system is the continuous measurement of scattered light in every well which can be correlated to the growing biomass over time. Over the cultivation time, the biomass signal (scattered light) is increasing due to the cell proliferation. Different intensities in the rise of the biomass signals are observed because different clones have been cultivated.
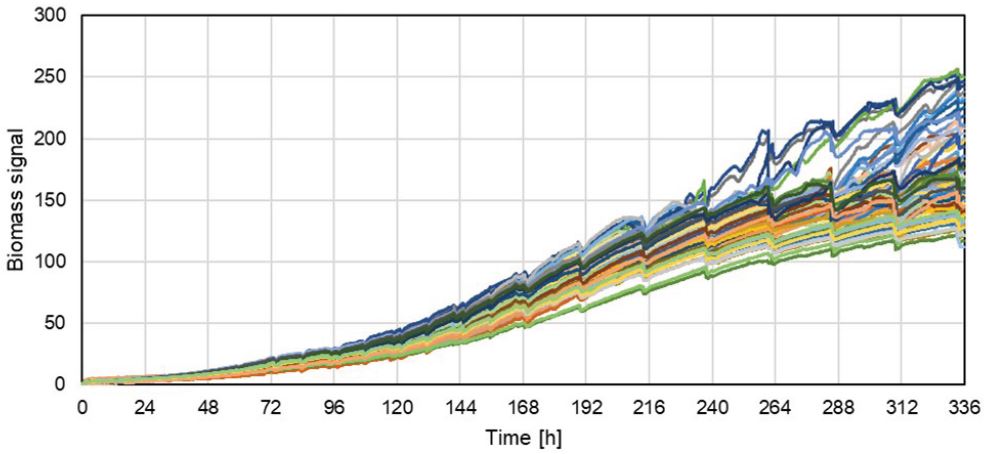
Figure 3. Biomass signal of 46 individual wells. Each line represents a single well/clone. Biomass signals over time are normalized to first biomass value to exclude the interference signal from the bottom of the plate.
As the Cydem VT system allows for daily automated cell count measurements, the biomass signal for selected wells was compared to the total cell density (TCD) that was determined with the integrated cell counter (Figure 4). According to the biomass signal, the chosen clones can be grouped into high (clone 45 and clone 12), mid (clone 42 and clone 3) and low (clone 46 and clone 24) proliferating cultures. The same trends are observed for the TCD measurement where clones are clearly ranked in the same order as for the biomass signal. These results demonstrate that monitoring of the biomass signal is an important indicator for proliferation rate of the cultured cells.
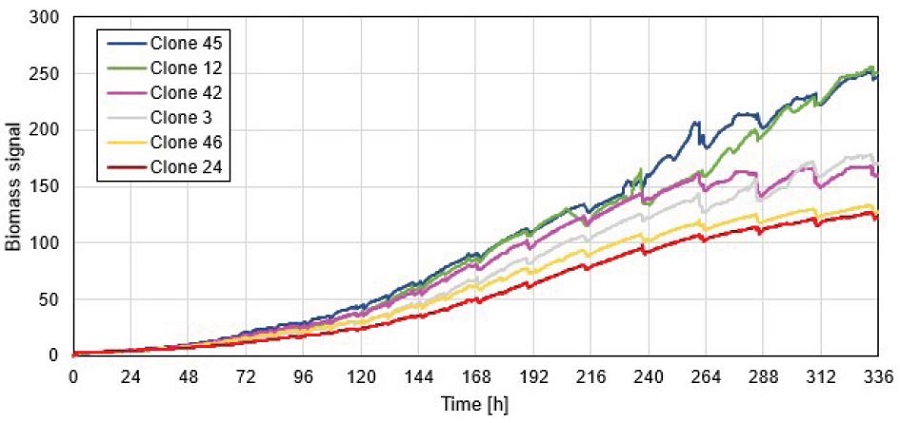
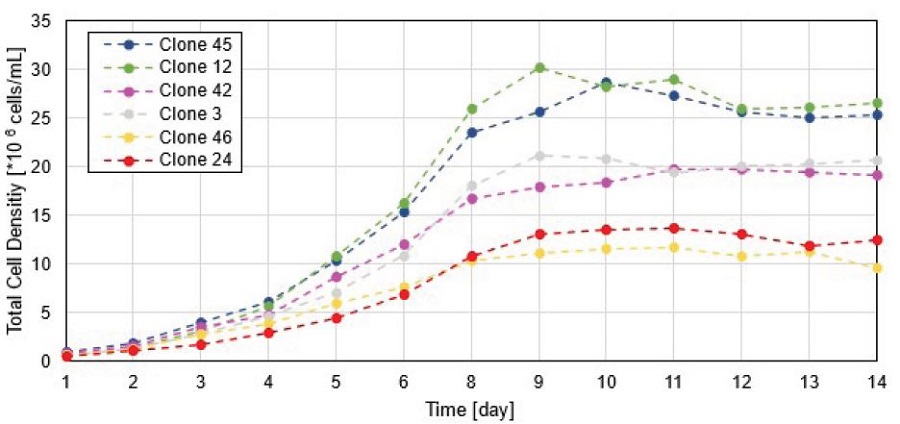
Figure 4. Comparison of biomass signal (upper graph) and measured TCD (lower graph) for selected clones. Biomass signals over time are normalized to first biomass value to exclude the interference signal from the bottom of the plate.
In addition to cell count measurements, Cydem VT system allows the measurement of antibody titer in all of the 96 wells. As described before, 46 different clones were cultured in duplicates in the presented experiment. IgG titer over the cultivation time is exemplarily shown for both replicates of four different clones (Figure 5). The antibody titers of the replicates show a good match demonstrating equal cultivation conditions even under individual gassing control of every well as replicates produce similar amounts of antibodies when cultured in the Cydem VT system.
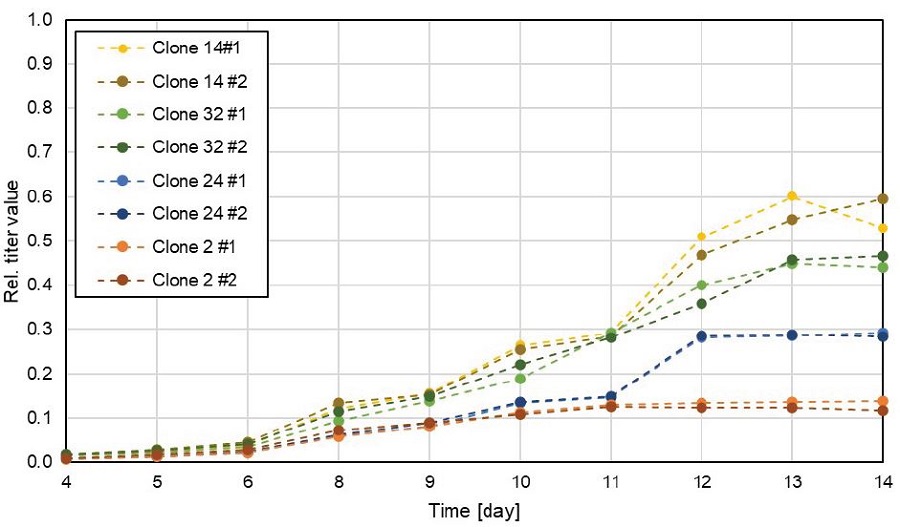
Figure 5. IgG titers of replicates measured with the Cydem VT system. Titer was measured daily from day 4 onwards with the integrated titer measurement module. IgG titer value is shown relative to the highest measured value over the course of experiment considering all 46 clones.
The accuracy of the titer measurement of the Cydem VT system compared to other devices for titer analysis was already demonstrated.1 However, samples on days 7, 10, and 14 were taken and measured offline with the Cedex Bio HT analyzer to focus on conformity between biological replicates (Figure 6). For that purpose, the measured IgG titers of all 46 pairs of replicates were analyzed against each other to determine the correlation between duplicates. In the graph, one dot represents the values for both duplicates whereas the IgG concentration of one bioreactor/well of the duplicate is plotted on the x-axis and the concentration of the other is plotted on the y-axis. The observed correlation of R2=0.9766 underlines again uniform culture conditions in the Cydem VT system.
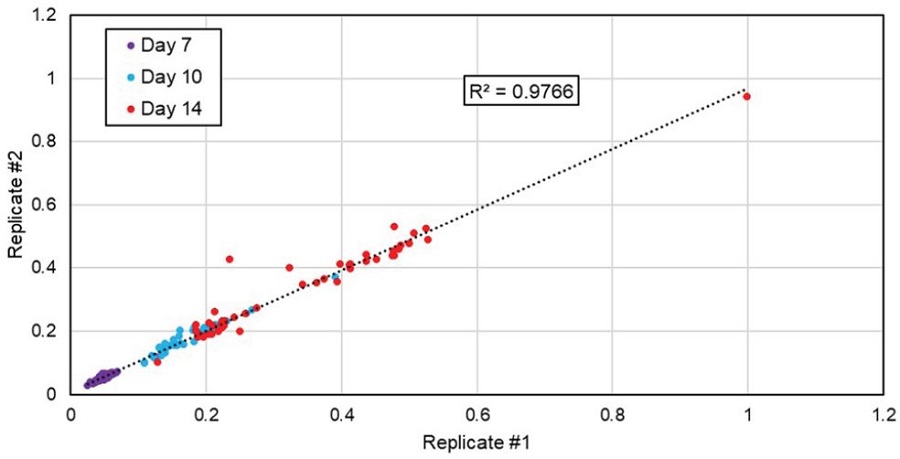
Figure 6. Correlation of titer production by replicates. 46 different clones were cultured in the Cydem VT system. Antibody concentrations were determined at indicated days with the Cedex Bio HT analyzer. Values for IgG titers were normalized to the highest value measured in this system over 14 days.
Next, data that were generated with a Cydem VT system were compared to data that were gathered with a DWFB or a 15 mL microbioreactor cell culture experiment. All experiments were performed simultaneously using the same sets of clones. Cell counts of the Cydem VT system were determined with the integrated module while data for antibody titer, the DWFB and the 15 mL microbioreactor cell culture were determined with the Cedex Bio HT device. Overall, clones cultured in the DWFB showed less viable cell density (VCD), and titer production compared to Cydem VT system or a 15 mL microbioreactor system (Figure 7). However, VCD and titer production are in a similar range for both automated cell culture systems.
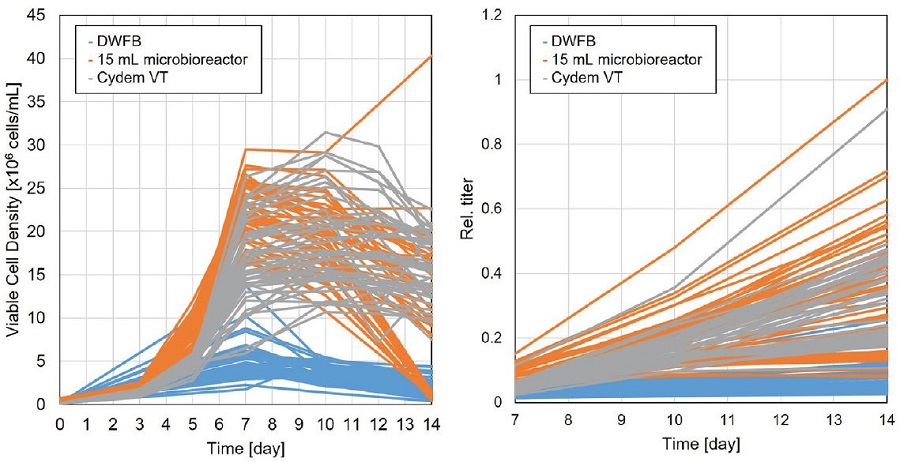
Figure 7. Comparison of viable cell density (left graph) and relative antibody concentration (right graph) of cells cultured in a DWFB, 15 mL microbioreactor system or Cydem VT system. Values for IgG titers were normalized to the highest value among systems over 14 days. All 46 clones are displayed. For Cydem VT system, one replicate of every clone is shown.
To evaluate differences of the three culture methods in more detail, VCD, viability and antibody titer of selected clones that are representative for the cultures are shown (Figure 8). Every clone showed a lower VCD and a faster decrease in viability when cultured as a DWFB compared to the 15 mL microbioreactor system or Cydem VT system. This is also reflected in the IgG concentrations where clones from the DWFB showed lower antibody production. Clone 3 showed higher VCD and a higher IgG production in the 15 mL microbioreactor system compared to Cydem VT system. In contrast, clone 5 showed an earlier decrease in VCD and viability when cultured in a 15 mL microbioreactor system compared to the same clone cultured in Cydem VT system. Also, the IgG concentration is higher when cells are cultured in the Cydem VT system than in a 15 mL microbioreactor for clone 5. However, clone 40 showed similar growth behavior and titer production in a Cydem VT system and in a 15 mL microbioreactor system.
Of note, this data highlights again the conformity of VCD, viability, and produced antibody between clone replicates in the Cydem VT system.
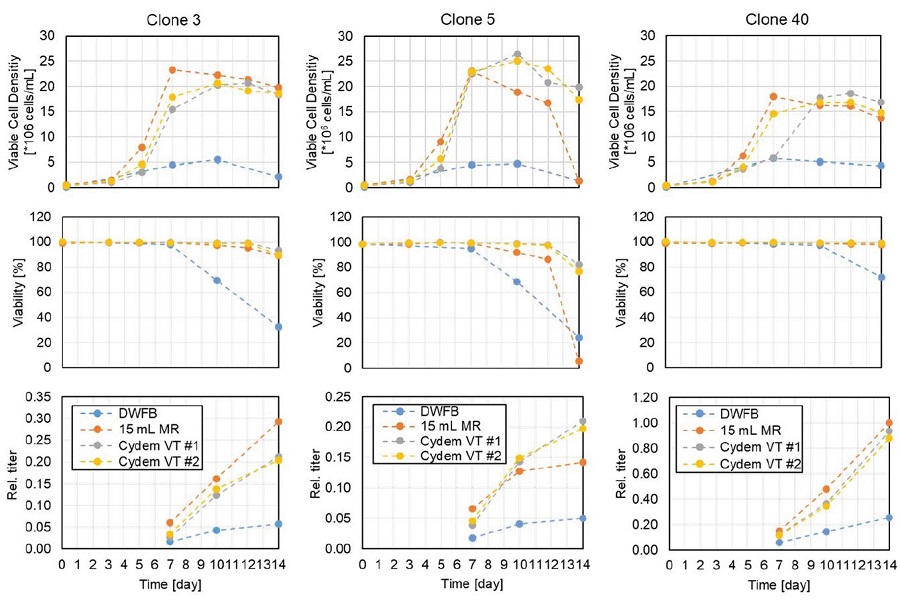
Figure 8. Comparison of Viable Cell Density, viability and IgG titer for selected clones cultured in a DWFB, 15 mL microbioreactor system (15 mL MR) and Cydem VT system. Values for IgG titers were normalized to the highest value among systems over 14 days. Clone replicates cultured in the Cydem VT system are represented as #1 and #2.
As the Cydem VT system is designed for clone screening purposes, the ranking of the top five titer producing clones on day 10 of each experiment were compared (Figure 9). Day 10 of the cultures was chosen for this comparison, as the viability of clones in the different systems was more similar compared to day 14 of the culture where clones cultured in the DWFB showed strong decrease in their viability. All experiments resulted in the identification of the same top-producing clone (clone 40). However, ranking of the following clones is more similar in the Cydem VT system and the 15 mL microbioreactor than in the DWFB culture. While Cydem VT system and the 15 mL microbioreactor system share the rank of their top 5 clones with a twist of rank 4 and rank 5, those clones were differently ranked in the DWFB culture. Differences in the clone ranking based on titer production between Cydem VT system and the DWFB is even more pronounced considering all 46 clones of the experiments. On the other hand, correlation of the 15 mL microbioreactor system and Cydem VT system gives better results.
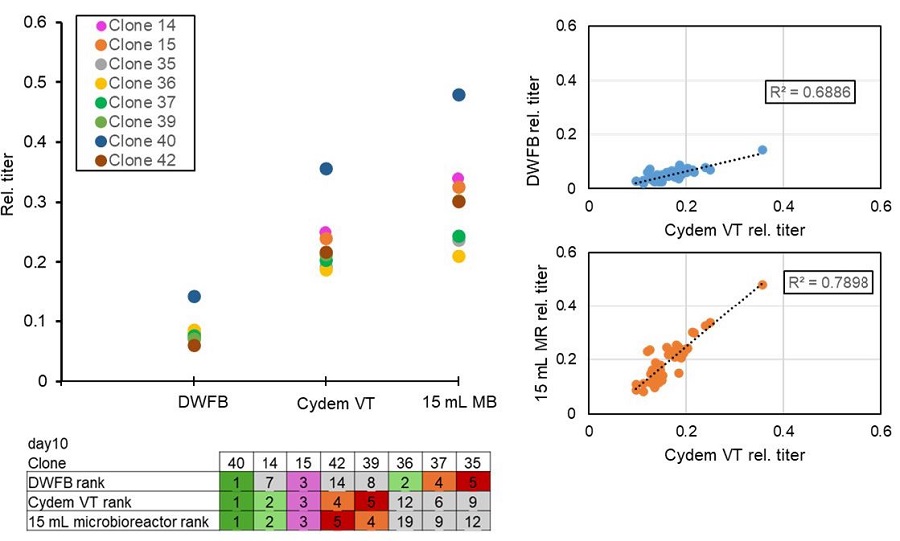
Figure 9. Clone ranking on day 10 based on produced IgG for the top 5 clones of each cell culture platform (left graph and table) and correlation of titer values of all 46 clones (right graphs) between the Cydem VT system and a 15 mL microbioreactor and DWFB experiment. Day 10 of the experiment was chosen because of better comparability between the clones, as viability was still on the same level. Values for IgG titers were normalized to the highest value among systems over 14 days.
Discussion
In summary, clones cultured in the Cydem VT system showed uniform cell growth and titer production between replicates. Differences in clone growth can be continuously monitored by biomass signal and pH profile where the latter can be balanced by individual gassing and base addition of every well.
Comparison of the Cydem VT Automated Clone Screening System with other clone screening platforms highlights that the Cydem VT system represents an excellent alternative to traditional cell culture in a DWFB. Further, clones showed similar growth and titer production compared to other automated cell culture systems, which facilitates scale-up studies. An integration of Cydem VT system into clone screening workflows supports valuable information of produced antibody titer and cell growth in early phases of the process thanks to automated measurements on a daily basis with minimal required manual lab work.
Acknowledgements
We would like to thank Bayer AG for providing cells and scientific support and discussions to successfully conduct and analyze the described experiments.
References
- Application Note: Efficient clone screening with increased process control and integrated cell health and titer measurements with the Cydem VT Automated Clone Screening System, 2024-GBL-EN-105878


