Migration of Panels Designed on the CytoFLEX S Flow Cytometer to CytoFLEX SRT Cell Sorter
Objective
Learn how to import settings, plots, and statistics from CytoFLEX S V-B-Y-R flow cytometer to the CytoFLEX SRT cell sorter
Introduction
Designing reliable flow cytometry antibody staining cocktails often requires a good deal of thought and effort to balance sensitivity of detection, minimizing spectral overlap, and availability of conjugated antibodies against the markers of interest. It is often desirable to perform initial design work for sorting experiments on flow cytometry analyzers due to easier access and the desire to use the sorter for experiments requiring sorting.
Moving multicolor cocktail experiments from an analyzer to a sorter does introduce some steps that can be tedious. Often, analyzers and sorters use different software, and experimental details such as complex gating strategies need to be repeated, taking additional time and introducing the possibility of mistakes.
CytoFLEX SRT uses CytExpert SRT software, a derivative of CytExpert used across the entire platform, for setup and analysis. This software match and the potential to parameter match the CytoFLEX S and CytoFLEX SRT instruments allows users to do design and analysis work on CytoFLEX S flow cytometer and import that experiment into their sorter with minimal manual steps.
In this application note, an experiment created in a CytoFLEX S flow cytometer will be imported into a parameter matched CytoFLEX SRT cell sorter. After checking gain and compensation settings, the same regions that were created in the analyzer will be used as sort regions in a multicolor sort experiment.
Parameter Matched Detector Configuration
The detector configuration for CytoFLEX SRT cell sorter is an extension of the CytoFLEX S V–B–Y–R flow cytometer. The CytoFLEX SRT contains two additional channels, one off of the Yellow Green laser and another off of the Violet laser. Having both instruments enables high volume laboratories to design a sort gating strategy using the analyzer before executing on the sorter.
| Platform | Blue (488 nm) | Red (638 nm) | Yellow Green (561 nm) | Violet (405 nm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CytoFLEX SRT | 525/40 | 690/50 | 660/10 | 712/25 | 780/60 | 585/42 | 610/20 | 675/30 | 710/50 | 780/60 | 450/45 | 525/40 | 610/20 | 660/10 | 780/60 |
| CytoFLEX S V-B-Y-R Series | 525/40 | 690/50 | 660/10 | 712/25 | 780/60 | 585/42 | 610/20 | 690/50 | 780/60 | 450/45 | 525/40 | 610/20 | 660/10 | ||
Figure 1. Comparison of Fluorescence Detectors. The detector channels for both the CytoFLEX SRT (top row) and the CytoFLEX S V-B-Y-R flow cytometer (bottom row) are compared. The CytoFLEX SRT contains two additional channels, one from the Yellow Green laser and one from the Violet laser
CytExpert templates can be ported to CytoFLEX SRT to save time on instrument setup.
Exporting an .xit file from CytExpert software
In this example, data from a CytoFLEX S flow cytometer was generated using a DURAClone IM T cell subset kit according to the instructions for use. For the initial setup and analysis the sample used was the Abnormal cell control of the ClearLLab assay. This allowed for multiple trials with a sample of known properties.
Following the gating and analysis suggestions in the instructions for use, multiple cell types can be analyzed with varying levels of expression of the markers in the unitized DURAClone dry antibody panel.
If Electronic Record Management is not installed, navigate to the folder where you have stored your experiment. By default this is C:\Users\xxxxxxxx\Documents\CytExpert Data\.
Locate the .xit (CytoFLEX experiment file) and its associated file folder where .fcs files are stored.
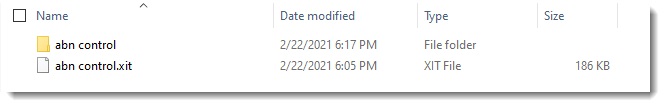
Figure 2. Required Folder Structure.CytExpert expects the fcs files to be stored in a folder with the same name as the xit file.
IMPORTANT: the .xit file and the file folder both must be present in the same folder for conversion to complete successfully.
If it isn’t desirable to transfer the .fcs files, save a copy of the experiment and remove the tube files from the copy.
Move this file to your portable drive or however you would like to transfer the files. Save the file on the hard drive of the CytoFLEX SRT workstation.
If Electronic Record management (ERM) in installed, .xit files and their associated folders have scrambled names. To retain the information, export the .xit files and folders needed using the Export Experiment option in the Experiment Explorer.
IMPORTANT: For files to successfully export, the experiment must not be open. If the experiment you wish to export is open select “Close Experiment” from the file menu.
Converting an .xit file into an .xits Experiment in CytExpert SRT software
Open CytExpert SRT software. From the file menu select Convert CytExpert Experiment. Using the top “browse” button locate the .xit file you wish to convert. Use the bottom “browse” button to navigate to the location you would like to store the .xits file. By default these are stored at C:\Users\xxxxxxxx\Documents\CytExpert Data\
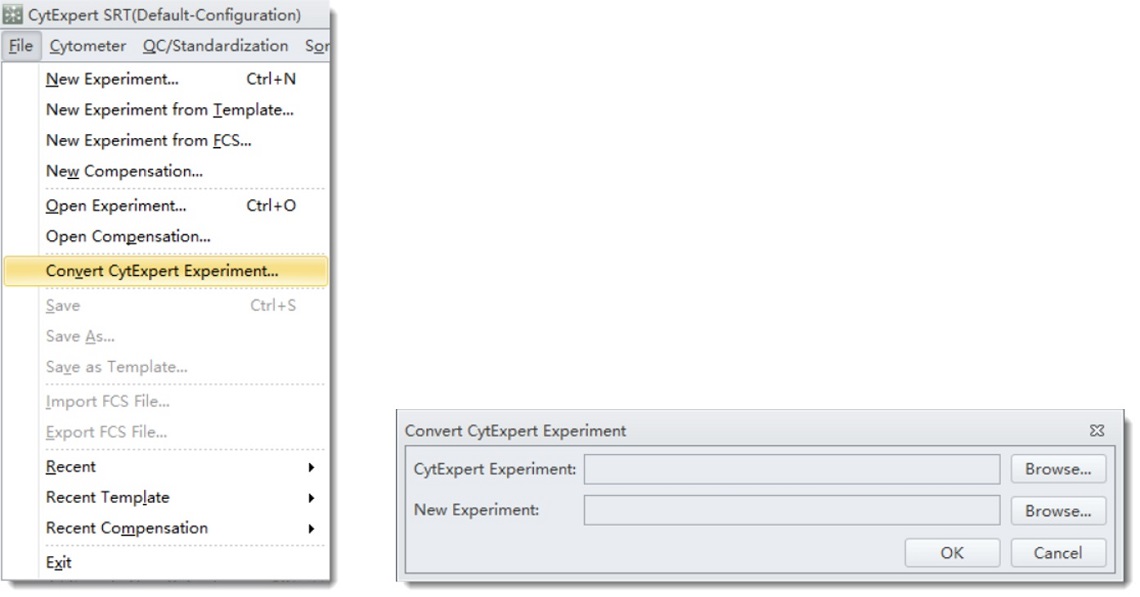
Figure 3. Convert CytExpert Experiment.Series of menues requried for conversion of CytExpert file to CytExpert SRT file.
The figures below show the same experiment before and after conversion.
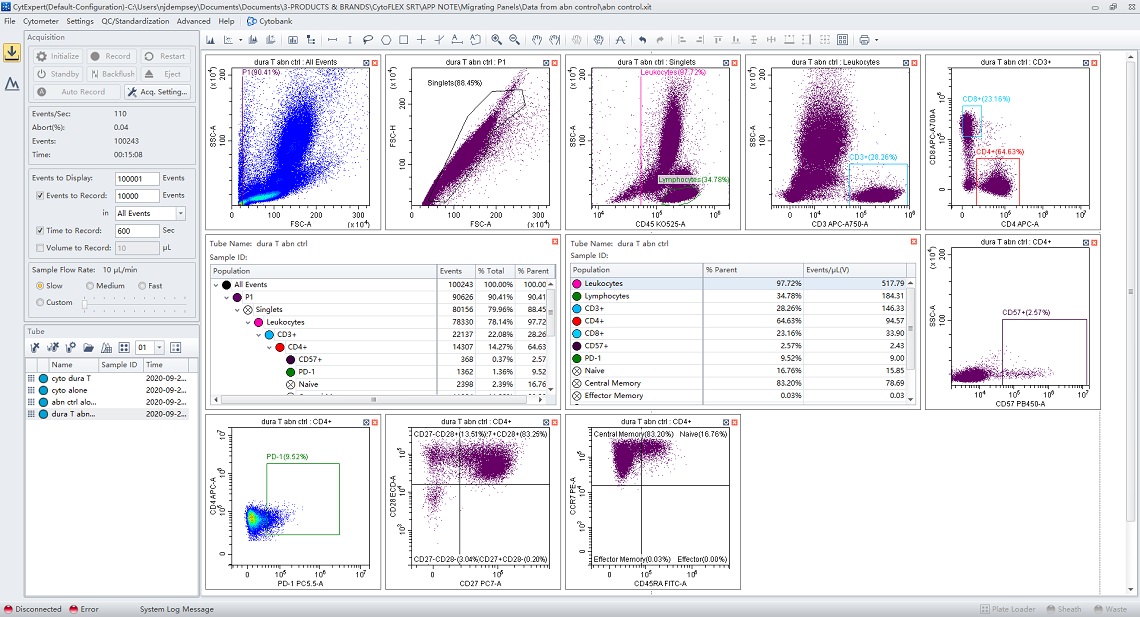
Figure 4. Data Displayed in CytExpert.Data was acquired on a CytoFLEX S V-B-Y-R instrument. Plots, gates, regions, and statistics were created and applied to four samples.
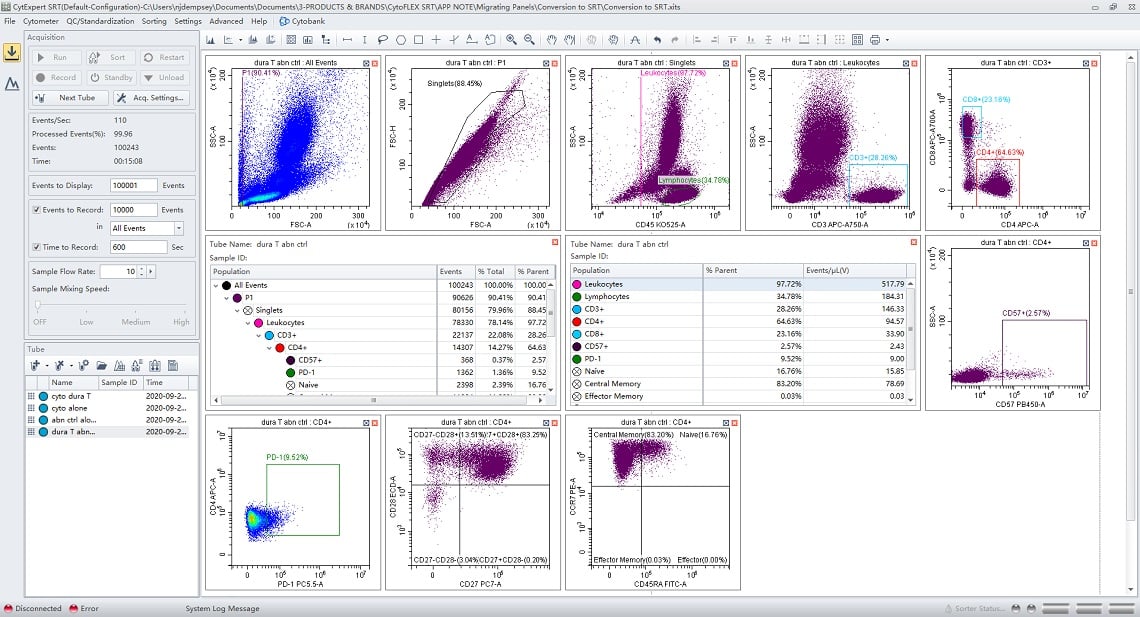
Figure 5. Converted Data Displayed in CytExpert SRT. Files shown in figure 5 were converted into CytExpert SRT files. All plots, gates, regions, and statistics tables have been converted and displayed in the interface. The Tube workspace shows all samples from the original file have been ported over.
Although the CytoFLEX SRT cell sorter and the CytoFLEX Platform of analyzers are very similar, changes in gain and/or compensation may be needed to place populations in the desired locations on the plots. The standardization workflow available on the CytoFLEX Platform can be used to adjust gains to place reference material fluorescence in the same location on the two instruments. For the best compensation performance, running compensation controls on the CytoFLEX SRT and applying them to the converted experiment is recommended.
Making use of the CytoFLEX Platform’s Compensation Library feature, these compensation controls can be run at any time and stored. Compensation can then be converted based upon the new gains applied after conversion and standardization.
For more details on Standardization in CytoFLEX Platform instruments please see the Instructions for Use for the CytoFLEX Platform or the CytoFLEX SRT Cell Sorter.
TIPS FOR SUCCESS
- Make certain that parameters match for the easiest experience. Matching means the same laser line for excitation and channel name. Channels that do not match will be blanked and regions will need to be redrawn.
- The CytoFLEX S V–B–Y–R flow cytometer has the same 4 lasers as the CytoFLEX SRT and has the most potential for experiment conversion.
Summary
The CytoFLEX S V-B-Y-R flow cytometer and the CytoFLEX SRT cell sorter have similar fluorescence detector configurations. This makes designing panels and optimizing experimental conditions using the analyzer easier. Indeed, the software for the sorter, CytExpert SRT, includes functions that allow CytExpert files to be imported. This saves operators time and effort when they are ready to conduct cell sorts based on the optimized conditions.
When acquiring data using the plots, gates, and regions established on the CytoFLEX S V-B-Y-R flow cytometer, it will be necessary to assess the instrument settings. Changes in the gain settings or the compensation matrix may be required. Use of the Standardization Tools in the platform can help to adjust the data display. Once compensation is applied all that remains is selection of populations of interest and the application of sort logic and stopping conditions.
For Research Use Only. Not for use in diagnostic procedures.


