Automating Genomic DNA Extraction from Whole Blood and Serum with GenFind V3 on the Biomek i7 Hybrid Genomic Workstation
Content Type: Poster
Authors: Randy Pares, M.S.; Li Liu, Ph.D.; Han Wei, Ph.D.; Mary Blair
Abstract
The isolation of high quality genomic DNA (gDNA) is the precursor to many molecular biology assays. The new GenFind V3 Blood and Serum DNA Isolation Kit from Beckman Coulter uses the patented SPRI (Solid Phase Reverse Immobilization) paramagnetic bead technology to isolate genomic DNA from fresh or frozen whole blood and serum containing Citrate, EDTA, or Heparin anticoagulants, as well as from cultured cells. GenFind V3 uses an improved cell lysis buffer and Proteinase K treatment to rupture cell membranes and digest proteins to give consistently high quality gDNA with improved yields and purities. The GenFind V3 kit supports up to 400 µL sample input volumes from blood or serum or two million cultured cells and can be performed in either a 96-well plate or tube-based format. Additionally, the need for large volume whole blood extractions continues to grow along with the ability to easily automate the process to alleviate user errors. To address this need, a protocol that supports >400 µL and up to 2 mL of whole blood is available that can also be performed in an automated fashion using a 24-well plate. Volumes greater than 2 mL can be performed in a tube-based format. Currently, we are also in the process of integrating a Thermo Scientific KingFisher™ Presto on the i-Series to allow for higher volume processing and faster throughput.
Here, we demonstrate a walk-away automated solution for GenFind V3 kit to purify up to 400 µL of whole blood using the Beckman Coulter Biomek i7 Hybrid Genomics Workstation. The Biomek i-Series method is a high yielding and robust nucleic acid purification process and can process up to 96 samples in a 96-well format in less than 3.5 hours with minimal user interaction and no off-deck centrifugation or vacuum filtration. The method is also compatible with the Biomek i-Series NGS workstation, facilitating the installation process for existing Biomek i-Series users.
Materials and Methods
Whole blood was collected from four individual donors in blood collection tubes coated with EDTA, Citrate, and Heparin anticoagulants. Four technical replicates for each donor were used and a total of 48 samples of 400 µL were aliquoted in a 2.2 mL Abgene deep well plate (16 EDTA, 16 Citrate, 16 Heparin). Genomic DNA was then extracted using the GenFind V3 Blood and Serum DNA Isolation automated method implemented on the Biomek i7 Hybrid platform. All extracted genomic DNA samples were analyzed using a NanoDrop One (Thermo Scientific). 15 samples (five EDTA, five Citrate, and five Heparin) were randomly selected and analyzed using 2200 TapeStation using the Genomic DNA ScreenTape kit (Agilent Technologies). Those same 15 genomic DNA samples were then converted to NGS sequencing libraries using the Kapa HyperPlus Kit implemented on the Biomek i-Series i5 NGS Workstation. NGS library QC was performed using the 2100 Bioanalyzer with the High Sensitivity DNA kit (Agilent Technologies).Results: Automated gDNA Extraction
Whole blood samples were used for genomic DNA extraction using the Biomek i7 Dual Hybrid Genomic Workstation. Genomic DNA was quantified using NanoDrop One (Figure 1) and size distribution (Figure 2) and DNA Integrity Numbers (DIN) was calculated with the Agilent TapeStation using Genomic ScreenTape (Table 1).
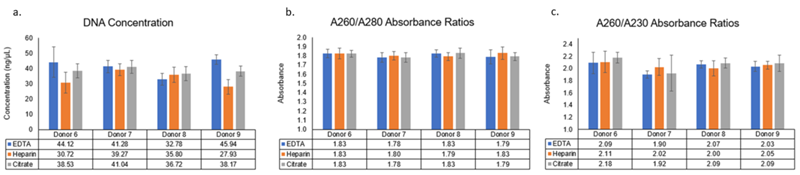
Figure 1: gDNA Concentration and purities determined using NanoDrop
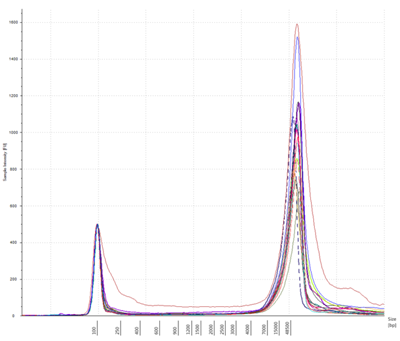
Figure 2: gDNA size distributions for 16 randomly selected samples from the Agilent TapeStation
| Donor | Blood Tube Type | DIN |
|---|---|---|
| D6 | EDTA | 9.6 |
| D6 | Heparin | 9.5 |
| D6 | Citrate | 9.6 |
| D7 | EDTA | 9.2 |
| D7 | Heparin | 9.4 |
| D7 | Heparin | 9.6 |
| D7 | Citrate | 9.5 |
| D8 | EDTA | 7.8 |
| D8 | EDTA | 9.1 |
| D8 | Heparin | 9.6 |
| D8 | Citrate | 9.5 |
| D8 | Citrate | 9.2 |
| D9 | EDTA | 9.5 |
| D9 | Heparin | 9.6 |
| D9 | Citrate | 9.2 |
Table 1: DIN for 16 randomly selected samples from the Agilent TapeStation
Biomek i-Series Automation
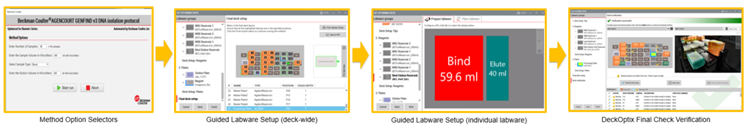
The Biomek i-Series platform represents the newest automated liquid handler from Beckman Coulter. The workflow described below was performed on a Biomek i7 Dual Hybrid equipped with a 1200 µL multichannel pod and a 1 mL Span-8 pod. The instrument was equipped with an orbital shaker and a shaking peltier, and has the option of an integrated Thermo Scientific Automated Thermocycler (ATC). The Biometra TRobot thermocycler can also be integrated as a substitution to the ATC. Biomek Method Launcher combined with the HTML Method Option Selector and Guided Labware Setup allow for ease of operation and flexibility in workflow scheduling. A final check of the deck is made using DeckOptix to reduce the number of costly errors that can be made during setup.

Results: Automated NGS Library Preparation
15 genomic DNA samples and one genomic DNA control (Coriell Human Reference DNA) were prepared into NGS sequencing libraries using the Roche KAPA HyperPlus Library it using the Biomek i5 NGS Workstation. Prepared libraries were analyzed with the Agilent 2100 Bioanalyzer using a High Sensitivity DNA kit (Figure 3).
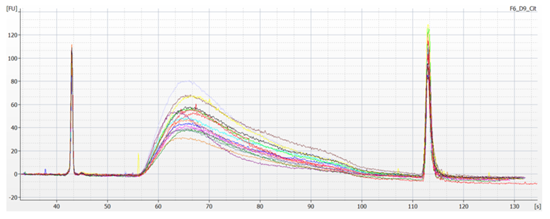
Figure 3: Bioanalyzer trace for 15 NGS libraries prepared from gDNA samples
Conclusions
In conclusion, we have shown that automation of GenFind V3 Blood and Serum DNA Isolation Kit on the Biomek i7 Genomics Workstation delivers high quality gDNA samples that are ready for sequence ready libraries and downstream NGS applications. Future directions for automation of GenFind V3 include standardized integration of the Thermo Scientific KingFisher™ Presto on i-Series for higher sample input.
Roche KAPA HyperPlus Kit is For Research Use Only. Not for use in diagnostic procedures. https://sequencing.roche.com/en/legal.html
Beckman Coulter makes no warranties of any kind whatsoever express or implied, with respect to this protocol, including but not limited to warranties of fitness for a particular purpose or merchantability or that the protocol is non-infringing. All warranties are expressly disclaimed. Your use of the method is solely at your own risk, without recourse to Beckman Coulter. Not intended or validated for use in the diagnosis of disease or other conditions. This protocol is for demonstration only, and is not validated by Beckman Coulter. Biomek i-series are not labeled for IVD use and are not intended or validated for use in the diagnosis of disease or other conditions. © 2019 Beckman Coulter, Inc. All rights reserved. Beckman Coulter, the stylized logo, and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries. All other trademarks are the property of their respective owners.

