Changes to USP <1788> Subvisible Particulate Matter
A revised chapter <1788> became official on May 1, 2021. This paper discusses some of the new content. <1788> is an informational chapter complimenting chapters <787>, <788>, <789> and <1787>. For the first time Flow Imaging is introduced as a complimentary, orthogonal test to the HIAC Light Obscuration compendial pass/fail test for injectable drug products.
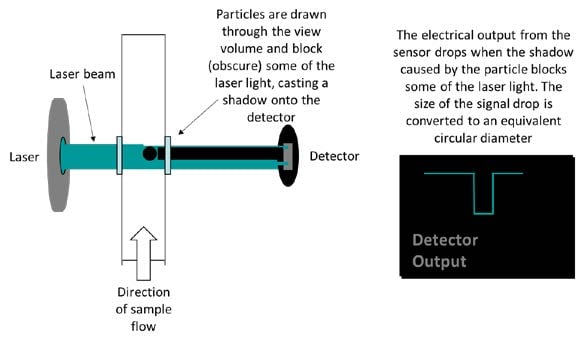
Fig. 1: The HIAC Light Obscuration technique
Although Flow Imaging can provide more information than the HIAC Light Obscuration test, this chapter points out that there have been decades of product quality test data collected using the HIAC Light Obscuration test method and, as such, it remains the primary compendial pass/fail test method in chapters <787>, <788> and <789>.
This chapter references USP<1787> as a good source of orthogonal test methods for forensic investigation of sub-visible particle load. Tracking and identifying particles supports investigations into the source of the particles and can help drive product improvement and provide root cause information should a sample fail the HIAC Light Obscuration test.
Although the compendial pass/fail tests in <787> and <788> reference limits for the number of particles at ≥10μm and ≥25μm, this revised chapter suggests there is value in evaluating the particulate load in the sub-10micron range and recommends that routine testing using the HIAC Light Obscuration method be extended to investigate particle loads down to 2μm, suggesting adding measurements at ≥2μm and ≥5μm, pointing out that measurements at 2μm are a useful indication of the quantity of protein aggregates in biologics. As the chapter for opthalmics, <789>, also requires particle sizing and counting at ≥50μm, it also suggests data in the ≥100μm range is useful too. It goes on to suggest calibration of the HIAC using traceable spherical particles at 2, 5, 10, 15, 25, and 50μm sizes.
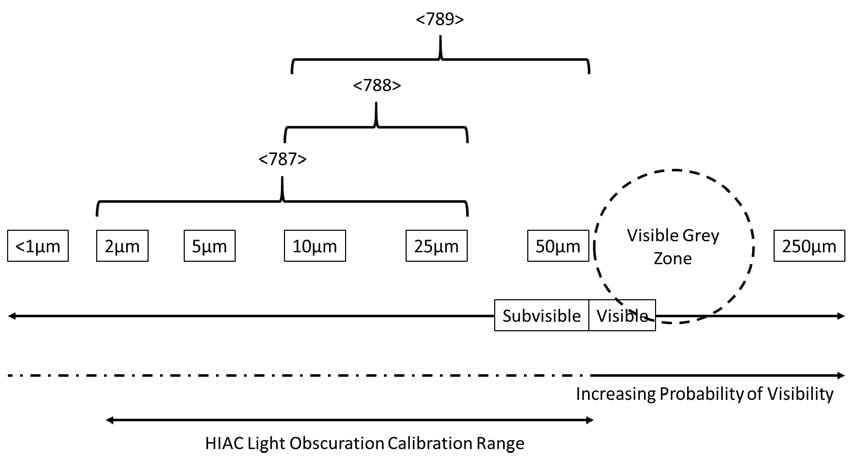
Fig. 2: Recommended Measurement and Calibration Ranges
Recommending a strategy of a holistic use of orthogonal test methods right from drug development through to final product QC in production, this revised chapter suggests that users should build a library of reference particle data to help technicians with particle classification, risk assessment and identification.
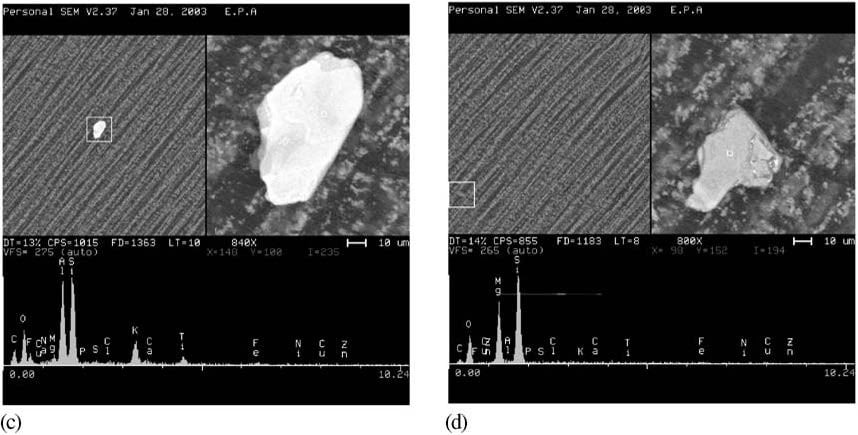
Fig. 3: Examples of Particles Seen Under a Scanning Electron Microscope
Complimenting the HIAC Light Obscuration method, Flow Imaging provides additional data allowing particle classification according to morphology and the Microscope Method can provide an isolate for further testing after counting is completed using an integrated approach to fully characterize and possibly identify particulate matter using light microscopy, vibrational spectroscopy, elemental analysis, and electron microscopy. Readers are recommended to reference USP<1787> for more information.
Although Flow Imaging and Microscope Method are recommended as orthogonal test methods to the HIAC Light Obscuration method, the revised <1788> chapter is clear that reported particle sizes will vary between these methods due to the different measurement technologies used. In each case the imperfect method of using two-dimensional information to describe a three-dimensional object is bound to lead to variable results being reported, with further complications arising in samples containing heterogeneous mixtures of particles. Chapter <1787> recommends that Coulter Counter and Laser Diffraction are used alongside the HIAC Light Obscuration test to gain more information on particle size and distribution.
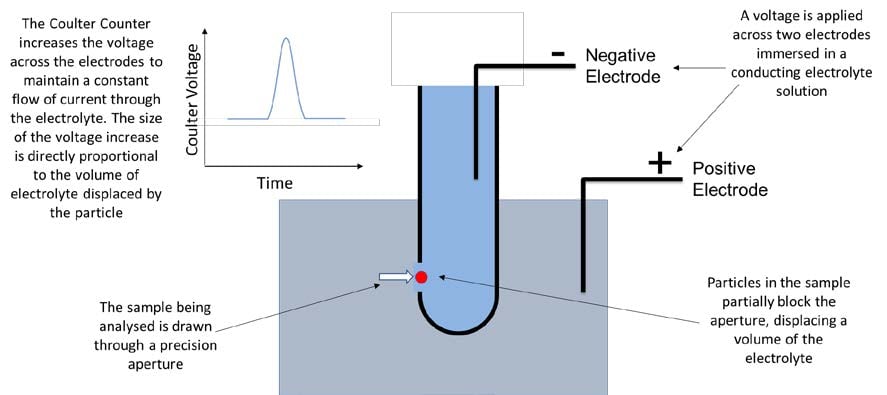
Fig. 4: The Coulter Counter Principle
The Coulter Counter provides not only equivalent spherical diameter, but also particle volume information whereas Laser Diffraction provides a wide dynamic measurement range in one analysis. The Coulter Counter is very sensitive to small variations in particle diameter because the volume measured is proportional to the cube of the particle radius, i.e. volume = 4/3πr3.
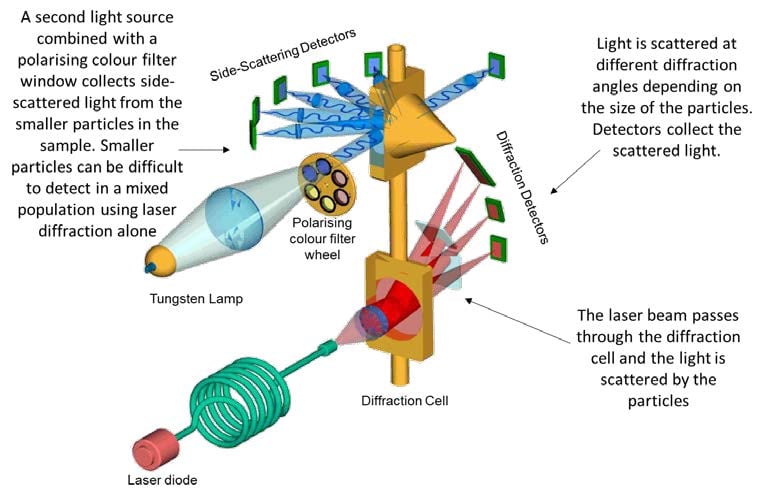
Fig. 5: Laser Diffraction
Laser diffraction combined with polarized intensity differential scattering can provide particle sizing distribution information for samples containing heterogeneous particle populations, which can prove challenging using laser diffraction alone where smaller particles may go undetected in the presence of populations of larger particles.
<1788> outlines the Instrument Standardization Test requirements and also System Suitability Testing, referring the reader to <1058> for more details. <1058> suggests that System Suitability Tests should be performed along with every sample analysis to ensure that the system's performance is acceptable at the time of the test. For particle counting and sizing tests, <1788> recommends routine testing using microsphere test standards and background count check using either filtered drug product or water blanks.
Helpful Links
HIAC Liquid Particle Counters
HIAC 9703+ Pharmaceutical Particle Counter
HIAC 8011+ Industrial Particle Counter
USP <787>
Light Obscuration
Subvisible Particulate Matter in Therapeutic Protein Injections
Particulate Testing Solutions for Contamination Control

