Automated Research Flow Cytometry Workflow Using DURA Innovations Dry Reagent Technology with the *Biomek i7 Automated Workstation and *CytoFLEX LX Flow Cytometer
In this paper you will
| Learn about DURA Innovations dry reagent technology and see how dry reagents can be used in an automated flow cytometry workflow | Discover an automated workflow solution for flow cytometry using an integrated CytoFLEX LX flow cytometer with the Biomek i7 Liquid Handling Automated Workstation |
Introduction
One of the challenges researchers encounter when utilizing flow cytometry is the lack of assay reproducibility and rigor that can occur during longitudinal studies and with assay transfer between sites or due to inter-person variability within a site. Sample preparation for high complexity flow cytometry assays are laborious, and errors can be introduced at several steps including sample dispensing and antibody cocktailing. The DURA Innovations dry reagent technology is a proprietary manufacturing process for making dried (not lyophilized) antibody cocktails that are stable at room temperature and are ready for addition of sample. These dry unitized reagents can shorten time-consuming protocols and help eliminate sources of human error.
The DURA Innovations dry reagent technology is available in off-the-shelf, expert designed *DURAClone antibody panels. Alternatively, user-defined dried antibody cocktails can be manufactured through our *LUCID Custom Design Services. In this application note, we showcase the dry reagent technology with an example of a cell count and viability assay as well as an example using an antibody panel of backbone markers for T-cell identification using the LUCID Custom Design Services. These two studies demonstrate the reproducibility and rigor that can be established by automating sample preparation and serve as examples of a streamlined automated workflow for sample preparation and acquisition.
We will describe the process of fully automating these two flow cytometry assays, in 96-well format, using a Biomek i7 liquid handling automated workstation integrated with a CytoFLEX LX flow cytometer and a microplate centrifuge (Figure 1A, 1B). This hands-free integrated system performs all steps involved in sample preparation, including plate shaking and centrifugation steps, and includes the automatic loading of the stained 96-well plate into the integrated CytoFLEX LX flow cytometer for acquisition (Figure 1D). In the first assay example, a dried panel of CD45-FITC, 7-AAD and fluorescent count beads was used to demonstrate a fully automated, robust and simplified workflow for assessing PBMC cell count and viability.
In the second example, we describe a fully automated assay combining dried down antibodies for gating T-cell populations together with liquid drop-in antibodies for measuring T-cell activation and immune status. An additional liquid drop of ViaKrome 808 fixable viability dye is added to exclude dead cells in unstimulated and anti-CD3/CD28 stimulated PBMC cell cultures.
Materials
PBMC Samples
Whole blood was collected from healthy volunteers and processed to peripheral blood mononuclear cells (PBMCs) using a standard Ficoll density gradient centrifugation method. PBMCs were either tested fresh or were cryopreserved and thawed on the day of use.
Stimulated PBMC Samples
Cryopreserved PBMCs were thawed, washed with complete media (RPMI-1640 + 10% FBS + 1% Penicillin Streptomycin), transferred to wells of a 6-well tissue culture plate and rested overnight in complete media supplemented with 2ng/mL IL-2 in 37°C + 5% CO2 incubator. The overnight rested PBMCs were either left unstimulated or were stimulated using DynaBeads T-Activator CD3/CD28 recommended procedure and cultured for 3 days in 37°C + 5% CO2 incubator. On day 3, the DynaBeads were removed from the stimulated PBMCs by a magnet and both unstimulated and stimulated PBMCs were washed with RPMI-1640 media and cryopreserved in a liquid nitrogen storage tank for storage until day of assay.
Reagents
| *Reagent | Supplier | Catalog Number |
| Phosphate Buffered Saline | Gibco | 10010-023 |
| Histopaque 1077 | Sigma | 10771-500mL |
| RPMI-1640 Media | HyClone | SH30255.01 |
| FBS heat inactivated | Gibco | 10082-147 |
| Penicillin Streptomycin Solution | HyClone | SV30010 |
| Recombinant Human IL-2 IS | Miltenyi Biotec | 130-097-744 |
| Recovery Cell Freezing Medium | Gibco | 12648-010 |
| Dynabeads Human T-Activator CD3/CD28 | Gibco | 11161D |
| DuraClone IM Count Tube | Beckman Coulter | C00162 |
| ViaKrome Fixable Viability Dye IR 808 | Beckman Coulter | C36628 |
| IO Test 3 Fixative Solution | Beckman Coulter | A07800 |
| Anti-human CD25-PE | Beckman Coulter | IM0479U |
| Anti-human LAG-3-FITC | BioLegend | 369308 |
| Anti-human TIM-3-APC | BioLegend | 345012 |
| Anti-human CD279-PC5.5 (PD-1) | Beckman Coulter | B36123 |
*For Research Use Only. Not for use in diagnostic procedures.

DURA Innovations Dry Technology LUCID Antibody Cocktails
Cell Count and Viability in a 96 shallow well plate
| Specificity | Purpose | Clone | Fluorochrome | Laser | Filter |
| CD45 | Leukocyte gating | J33 | FITC | 488 nm | 525/40 |
| 7-Aminoactinomycin D2 (7-AAD) | Viability | NA | NA | 488 nm | 690/50 |
| Fluorescent beads | Cell count | NA | Multiple wavelengths* | 561 nm | 585/42 |
*Beads were acquired in the Y561-585BP (PE) channel

T-cell backbone dried reagent cocktail in a 96 deep well plate:
| Specificity | Purpose | Clone | Fluorochrome | Laser | Filter |
| CD45 | Leukocyte gating | J33 | Krome Orange | 405 nm | 525/40 |
| CD3 | T-cell lineage | UCHT1 | APC-Alexa Fluor 750 | 638 nm | 763/43 |
| CD4 | T-Helper cell lineage | 13B8.2 | PC7 | 561 nm | 763/43 |
| CD8 | Cytotoxic T-cell lineage | B9.11 | Alexa Fluor 700 | 638 nm | 712/25 |
Liquid drop-in antibodies:
| Specificity | Purpose | Clone | Fluorochrome | Laser | Filter |
| LAG-3 | Immune checkpoint | 11C3C65 | FITC | 488 nm | 525/40 |
| CD25 | T-cell activation | B1.49.9 | PE | 561 nm | 585/42 |
| TIM-3 | Immune checkpoint | F38-2e2 | APC | 638 nm | 660/10 |
| CD279 (PD-1) | Immune checkpoint | B36123 | PC5.5 | 561 nm | 710/50 |
Tips for success
High speed shake at 1000 RPM for 15 seconds counter clockwise and again for 15 seconds clockwise on the orbital shaker resulted in complete resuspension of dry reagent and mixing with PBMC samples. Micro centrifuge settings of 2000 RPM for 5 minutes for wash steps were used to pellet PBMCs and minimize and potential cell loss.
AUTOMATED METHOD #1 – PBMC Cell Count with Viability in Shallow 96 Well Plate (Stain/No Wash)
The CytoFLEX-Biomek i7 integrated workstation was programmed to pipette 100 µl of each PBMC samples into eight wells of a single column of the LUCID dried reagent plate containing CD45-FITC, 7- AAD and counting beads. Sample mixing was performed by an on-deck orbital shaker. Samples were then incubated for 15 minutes at room temperature followed by addition of 100 µL of PBS buffer to sample wells and a gentle mixing by the orbital shaker. The sample plate was then automatically loaded into the integrated CytoFLEX LX flow cytometer. Samples were acquired immediately on the CytoFLEX LX flow cytometer using a pre-programmed experiment in CytExpert software for cell count and viability measurements.
AUTOMATED METHOD #2 – Unstimulated and Stimulated PBMCs stained with a T-cell Backbone Dried Reagent Cocktail in 96 Deep Well Plate and Drop-in Liquid Reagents (Stain/Wash)
A CytoFLEX-Biomek i7 integrated workstation was programmed to pipette 100 µl of unstimulated and stimulated PBMC samples into eight wells of a single column of the LUCID dry reagent plate containing CD45-Krome Orange, CD3-APC-Alexa Fluor 750, CD4-PC7 and CD8-Alexa Fluor 700. Sample mixing was performed by an on-deck orbital shaker. The selective tip feature of the multichannel head was used to load a single tip and pipette a titer amount of the liquid antibody cocktail containing LAG-3-FITC, CD25- PE, TIM-3-APC into each individual sample well. Samples were mixed on the orbital shaker and incubated for 15 minutes at room temperature. An automated wash step was performed by pipetting 600 µL of PBS into wells and loading plate into the integrated Agilent microplate centrifuge. After centrifugation, the supernatant was pipetted off and ViaKrome 808 fixable viability dye was added to wells using selective tip feature. Samples were mixed on the orbital shaker and incubated for 20 minutes at room temperature. Samples were washed as previously described and 200 µL of fixative buffer was then added to wells, gently mixed, and subsequently automatically loaded into the integrated CytoFLEX LX flow cytometer. Samples were acquired on the CytoFLEX LX flow cytometer using a pre-programmed experiment in CytExpert software for the nine-color staining panel.
Figure 1A.
Figure 1B.
Figure 1C.
Figure 1D
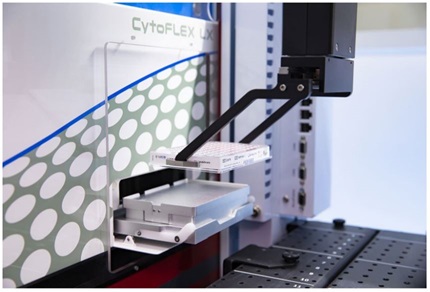
Figure 1: Integrated CytoFLEX-Biomek i7 workstation. A. The CytoFLEX is positioned on the left side of the system on a sliding platform that rotates to allow the user to easily switch between plate and tube mode without uncoupling the CytoFLEX from the integrated Biomek i7 workstation. B. Image shows the deck layout of the integrated CytoFLEX-Biomek i7 workstation. C. Image demonstrates the Biomek pipetting samples into a LUCID dried 96 well plate. D. Automated loading of a 96 well plate into the CytoFLEX LX.
Results
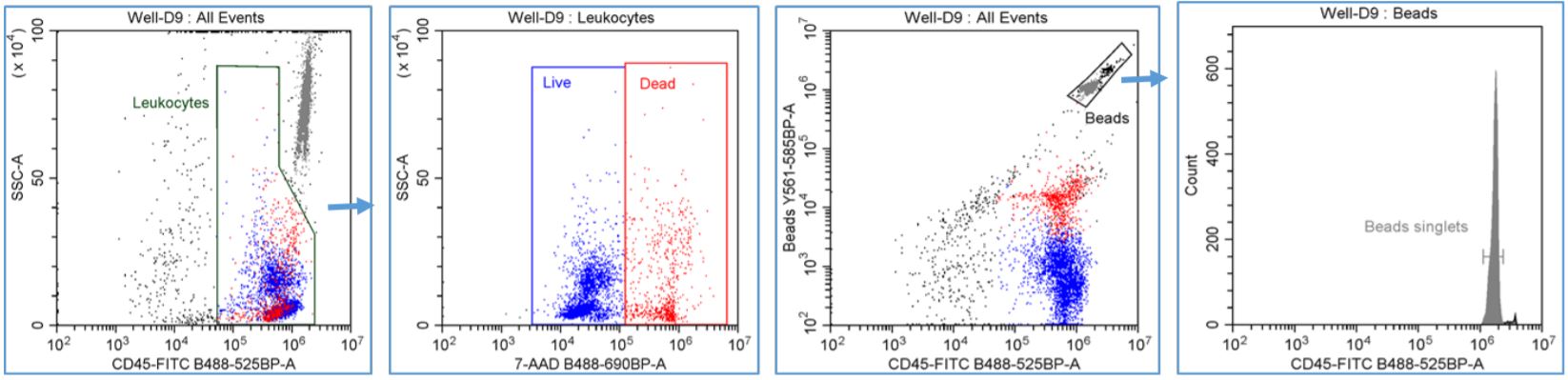
Figure 2. Automated PBMC Cell Count with viability - gating strategy: Leukocytes were gated using CD45-FITC vs. SSC-A dot plot and a region was drawn to encompass the CD45+ staining cells. LIVE and DEAD CD45+ leuokcytes were gated using SSC-A vs. 7-AAD dot plot with the Leukocytes gate applied and regions were drawn to emcompass the LIVE CD45+ (7-AAD -) and DEAD CD45+ (7-AAD +) leukocytes. Fluorescent counting beads were gated using the Y561-585BP channel vs. CD45-FITC dot plot and a region was drawn around the Beads population. The number of singlet bead count was measured using a FITC single parameter histogram with the Beads gate applied and an analysis cursor was drawn to encompass the single peak.
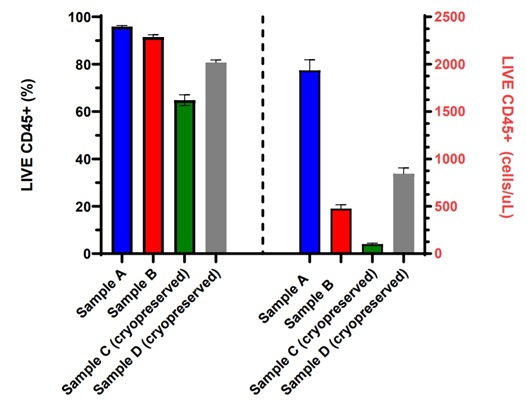
Figure 3. Fully Automated PBMC Cell Count with Viability: Fresh or cryopreserved PBMCs from four different samples were stained with CD45-FITC, 7-AAD and counting beads in the dried reagent format in a 96 shallow well plate using the Biomek i7 automation workstation and acquired on an integrated CytoFLEX LX flow cytometer. Data analysis was performed using CytExpert software. LIVE CD45+ (%) viable and LIVE CD45+ absolute cell counts (cells/µL) measurements for each of the eight replicates are displayed in a bar graph with error bars representing the standard deviation within each PBMC sample set.
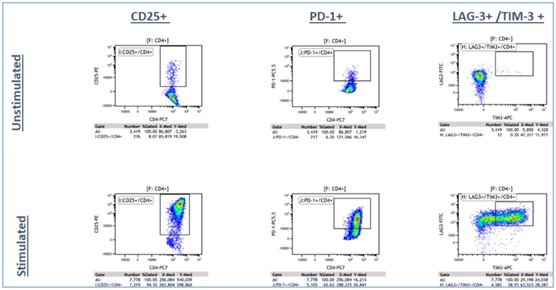
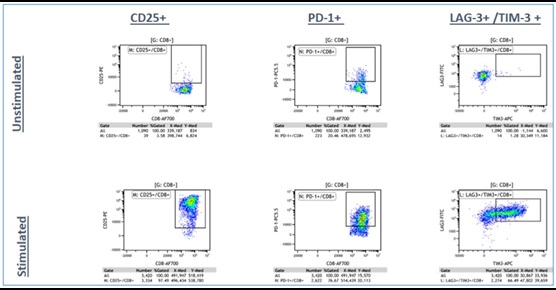
Panel A: LIVE CD3+/ CD4+ T-cells
Panel B: LIVE CD3+/CD8+ T-cells
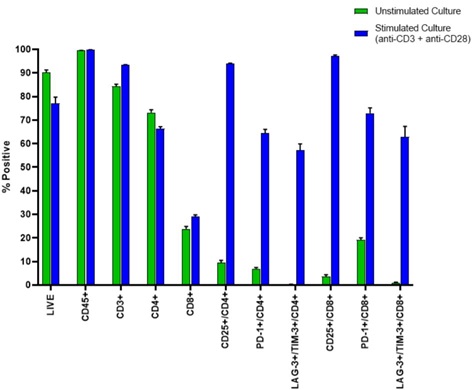
Figure 4a. Representative Data Plots from Unstimulated and Stimulated PBMCs Stained with an Antibody Panel of Backbone Markers for T-cell Identification and Drop-in Liquid Reagents using the CytoFLEX-Biomek i7 Integrated Workstation: Unstimulated and anti-CD3/CD28 stimulated PBMC (3 day) cultures were stained with CD45-Krome Orange, CD3-APC-Alexa Fluor 750, CD4-PC7 and CD8-Alexa Fluor 700 dried antibody cocktail with addition of liquid reagents LAG-3-FITC, CD25-PE, TIM-3-APC, PD-1- PC5.5 and ViaKrome 808 fixable viability dye, in 8 replicates each in a 96 deep well plate, using an automated stain/wash method on the CytoFLEX-Biomek i7 workstation. Wash steps were performed with an onboard integrated Agilent microplate centrifuge and samples were automatically acquired on an integrated CytoFLEX LX flow cytometer. Data analysis was performed using Kaluza data analysis software. Representative data plots for CD25+, PD-1+, and TIM-3+/LAG3+ populations are shown for both unstimulated and anti-CD3/CD28 stimulated LIVE CD3+/CD4+ T-cells (Figure 4a, Top Panel A) and LIVE CD3+/CD8+ T-cells (Figure 4a, Bottom Panel B).
Figure 4b. The percent positive for each gated population is shown in a bar graph for 8 replicates of unstimulated and anti-CD3/CD28 stimulated PBMC samples with error bars representing the standard deviation within each data set.
Automation of sample processing steps in flow cytometry assays can eliminate or decrease labor intensive manual processing steps and reduce human error. With the integration of the CytoFLEX LX flow cytometer and the Biomek i7 workstation together with DURAClone antibody panels, in 96-well plate format, we have demonstrated two different assays that show robust and reproducible data using a truly walk-away, fully automated workflow solution for flow cytometry. All together, an integrated system combined with dry antibody panels will reduce inter-person variability and increase assay rigor and reproducibility for longitudinal studies within a site or during assay transfers between different sites.
*For research use only. Not for use in diagnostic procedures.
© 2017 Beckman Coulter Life Sciences. All rights reserved. Beckman Coulter, the stylized logo, and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc. in the United States and other countries. All other trademarks, service marks products, or services are trademarks or registered trademarks of their respective holders.