Biomek i-Series Automated Illumina TruSeq® Stranded Total RNA Sample Preparation Kit Protocol
Biomek Automated Genomic Sample Prep Accelerates ResearchIntroduction
Illumina TruSeq® Stranded Total RNA Sample Preparation Kit protocol converts total RNA into a template library of known strand orientation (Illumina TruSeq Stranded Total RNA Library Prep Guide). First, rRNA and other high abundance transcripts are targeted for removal using a combination of biotinylated Ribo-Zero® probes and streptavidin removal beads (Illumina TruSeq Stranded Total RNA Library Prep Guide). Then the RNA is fragmented and copied into cDNA using a dUTP replacement strategy that maintains strand information in the library, followed by the addition of a single 'A' base and ligation of the adapter. The final PCR step enriches the products and creates the cDNA library for sequencing. The Illumina TruSeq Stranded total sample preparation Kit protocol enables analysis of both coding and noncoding RNA of known origin. Knowing the strand origin improves transcript annotation accuracy and alignment efficiency. Ribo-Zero probes have been developed targeting a variety of high-abundance transcripts for various species and tissues, including human, mouse, and rat rRNA, globins from blood-derived RNA, and plant rRNAs from plant RNA. In this technical note, we automate the Illumina TruSeq Stranded Total RNA Sample Preparation Kit protocol on Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation.
When compared to manual operations, The Illumina TruSeq Stranded Total RNA Library Prep Kit automated on Biomek platform provides:
- Reduced hands-on-time and increased throughput- Option to run the method end-to-end with only setup and tear-down touch points
- Reduction in pipetting errors
- Reduction of cost by using low reagent volumes
- Quick implementation with demonstrated methods
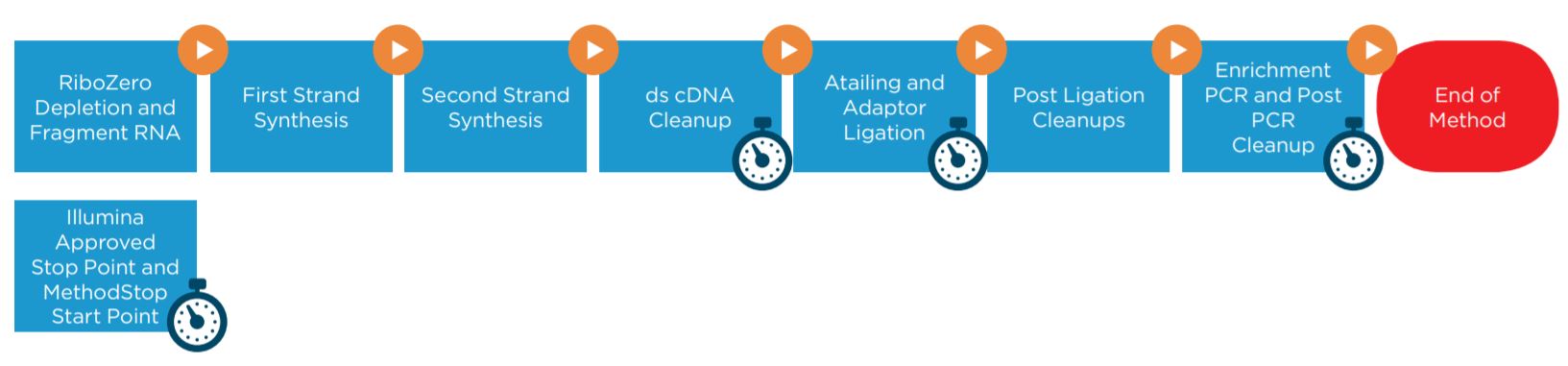
Figure 1. Illumina TruSeq Stranded Total RNA Sample Preparation Kit protocol
Automated method
Automation of Illumina TruSeq® Stranded Total RNA Sample Preparation Kit protocol provides efficient sample preparation and increased throughput with minimal hands-on time (Table 1). The automated method is designed to minimize exposure to hazardous reagents (e.g. Actinomycin D) by separately collecting the reagents for convenient disposal as biohazardous waste. Through the use of Biomek method launcher the users can easily implement and adapt the method to suit their needs.
| Process | Time | |
| 24 Samples | 96 Samples | |
| Prepare Reagents, Set up Instrument* | 15 mins | 30 mins |
| cDNA Synthesis | 3 hrs, 51 mins | 4 hrs, 32 mins |
| Library Construction | 2 hrs, 60 mins | 3 hrs, 46 mins |
| Total* | 7 hrs, 6 mins | 8 hrs, 48 mins |
*Timing estimate includes incubations and thermocycling. Timing estimate does not include reagent thawing
Table 1. Estimated run times for Illumina TruSeq® Stranded Total RNA sample preparation Kit protocol on the Biomek i7 Dual Hybrid Genomics Workstation.
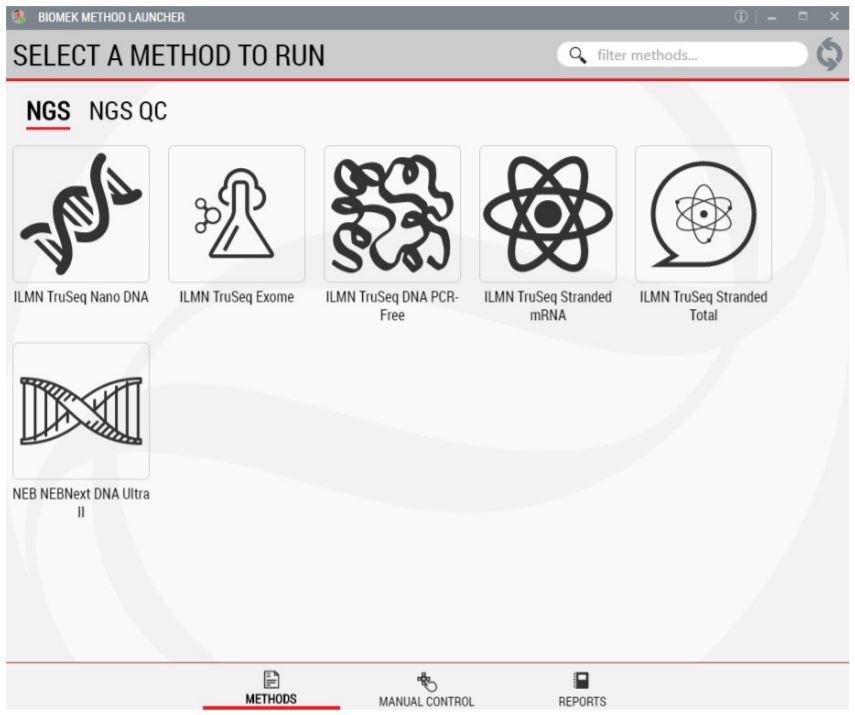
1. Biomek Method Launcher (BML): User friendly interface
BML is a user friendly interface for securely launching the method without introducing errors during method setup (Figure 2). Through BML, users can monitor the progress of the run, off site.
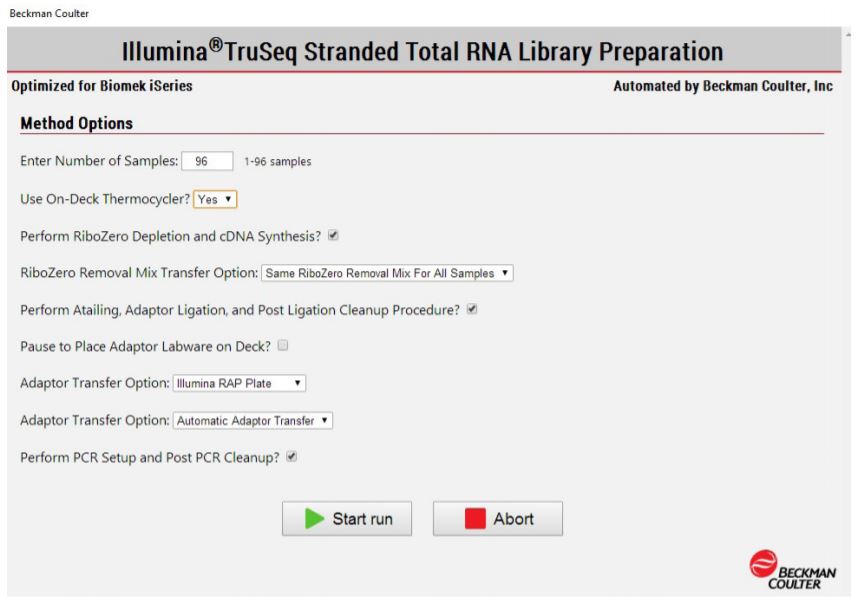
2. Method Options Selector (MOS): superior flexibility
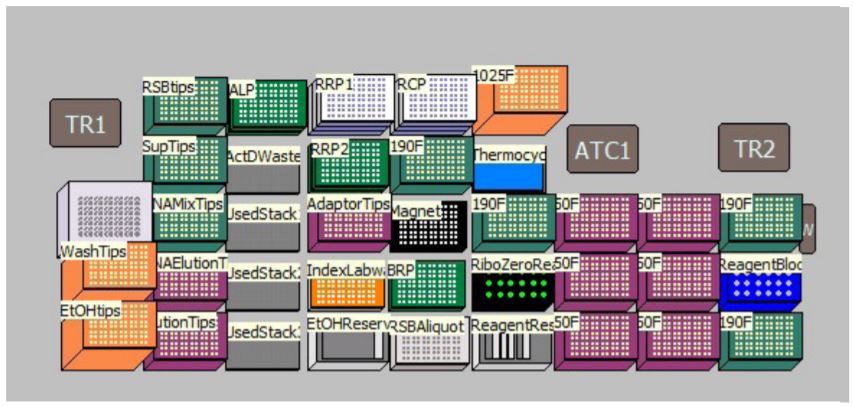
MOS provides several sample number and sample processing options (e.g. Ribo-Zero removal mix transfer options, adaptor transfer options, thermocycling options) to maximize flexibility of the method. MOS is arranged in a modular manner, providing workflow optimization (Figures 1, 3). Ability to start parts of the method based on the user constraints and logical start and stop points assigned based on Illumina's recommendations, allows users to recover from errors without having to start from the beginning of the method (Figures 1, 3). Users have the option to perform per-well Ribo-Zero depletion with different Ribo-Zero removal mixes or treat all wells with same Ribo-Zero Removal Mix. The thermocycling steps can be done either off-deck or on-deck using an automated thermocycler (ATC Thermo Fisher; Figure 4).
Figure 2. Biomek Method Launcher provides an easy interface to start the method
Figure 3. Biomek Method Options Selector indicates sample number and processing options
Figure 4. Deck Layout for TruSeq Stranded Total RNA sample preparation Kit protocol on Biomek i7 Dual Hybrid for 96 samples with on-deck thermocycling option
3. Guided Labware Setup (GLS): step by step instructions
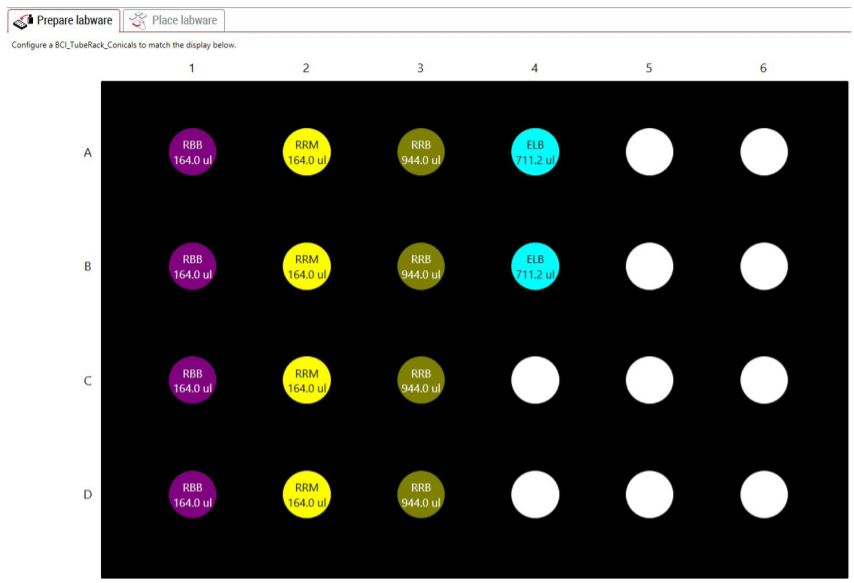
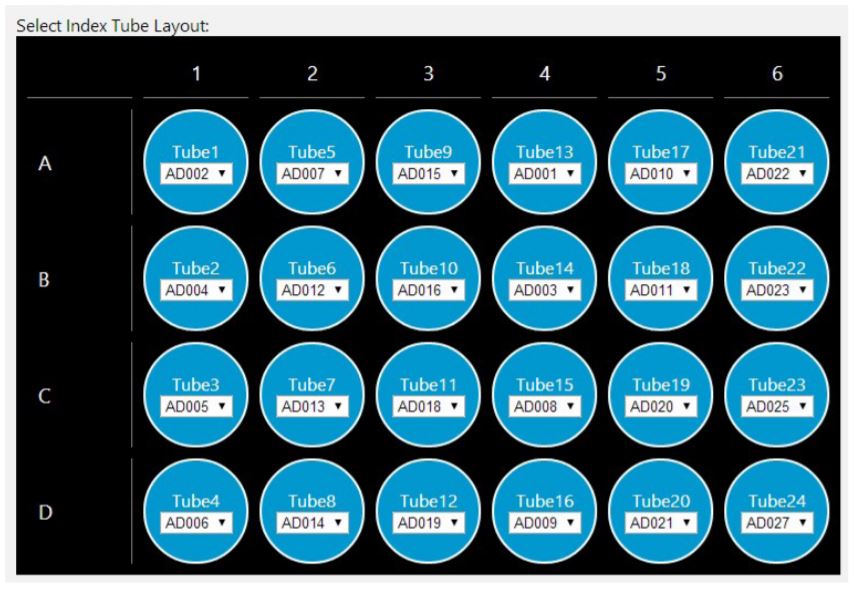
GLS provides the user specific step-by-step graphical setup instructions for reagent volume calculations, preparation of reagents and placement of labware on the deck (Figure 5). The GLS guides the users to place Illumina low throughput and high throughput adaptor labware on deck along with custom adaptor plates (Figure 6). The steps are generated based on the options selected in MOS. For instance, selecting automatic adaptor transfer creates dataset driven adaptor ID logs, indicating which adaptor has been assigned to which sample through Biomek software Data Acquisition and Reporting Tool (DART). DART gathers data and synthesizes runtime information from Biomek log files to capture all sample manipulations during the course of the method. Alternatively, users also have the option to customize adaptor assignments by uploading a .csv file. The method also supports placing Illumina LT and HT adaptor labware on deck along with custom adaptor selection. An optional pause step is included for placing adaptors on deck.
Figure 5. Guided Labware Setup indicates reagent volumes and guides the user for correct deck setup
Figure 6. Guided Labware Setup enables selecting index tube layout
Experimental design
Universal human reference total RNA (UHR 2 µg/µl, 1 µg/µl, 500 ng/µl, 100 ng/µl; 2 replicates from each) and RNA samples extracted using Beckman Coulter Agencourt RNAdvance Tissue Kit (Breast 58 ng/ul, Lung 156 ng/ul and Liver 29 ng/ul with 2 technical replicates each) was used for the automation of Illumina TruSeq® Stranded total RNA sample preparation Kit protocol on the Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation using 10 PCR cycles. After the preparation, the libraries were analyzed on Agilent TapeStation 2200 with Agilent High Sensitivity D5000 ScreenTape system.
Results
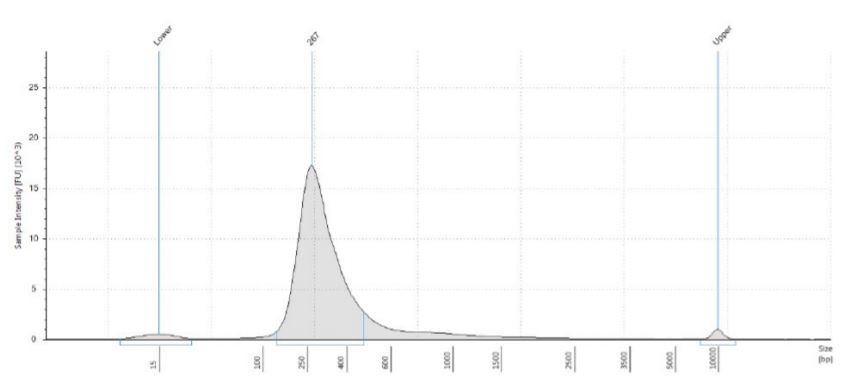
Agilent TapeStation results indicated that the majority of the prepared libraries are of expected size (Approximately 260 bp, TruSeq Stranded total RNA sample preparation Guide; Table 2; Figure 7). As indicated by Illumina, the cDNA yield increased with the amount of input RNA (Table 2).
| Sample ID | TapeStation size (bp) | Yield (pg/µl) |
| UHR 2 µg Rep1 | 267 | 11500 |
| UHR 2 µg Rep2 | 277 | 18200 |
| UHR 1 µg Rep1 | 271 | 4910 |
| UHR 1 µg Rep2 | 269 | 7170 |
| UHR 500 ng Rep1 | 266 | 3390 |
| UHR 500 ng Rep2 | 257 | 2880 |
| UHR 100 ng Rep1 | 279 | 988 |
| UHR 100 ng Rep2 | 271 | 742 |
| Breast Rep1 | 250 | 2360 |
| Breast Rep2 | 238 | 4050 |
| Lung Rep1 | 253 | 366 |
| Lung Rep2 | 257 | 451 |
| Liver Rep1 | 226 | 4010 |
| Liver Rep2 | 222 | 784 |
Table 2. Library quantification of automated Illumina TruSeq® Stranded Total RNA Sample Preparation kit protocol using Agilent TapeStation 2200
Figure 7. Electropherogram (Sample intensity vs. size in base pairs) of Agilent TapeStation corresponding to 2µg replicate 1 showing the libraries around expected size of the marker