ICH Q2 – the Challenge of Measuring Total Organic Carbon in Modern Pharmaceutical Water Systems
Abstract
Total Organic Carbon (TOC) is one of the quality attributes defined in the European and USA pharmacopoeias for pharmaceutical waters1. Modern water treatment systems can deliver such high purity water that TOC levels can be consistently close to zero and very difficult to measure with any accuracy. This paper discusses some of the challenges when using TOC analysers to demonstrate pharmacopoeial TOC level compliance for modern water systems in the light of the ICH Q2 document2 from the International Conference on Harmonisation.ICH Q2
In their ICH Q2 guidance document, Validation of Analytical Procedures2, the International Conference on Harmonisation highlights characteristics for consideration during the validation of the analytical procedures. It contains terms and definitions that are meant to bridge the differences that often exist between various compendia and regulators of the EC, Japan and USA. Users of TOC analysers to measure the impurities present in pharmaceutical grade waters may find the advice and guidance useful.
Image 1. The QbD1200 TOC Analyzer
TOC Analysis
TOC analysis in pharmaceutical grade waters is a non-specific test in that it effectively reports the weight in parts per billion (ppb) of carbon derived from organic material in the water, but it cannot discriminate from different types of organic material. In addition it cannot report the actual amount of organic material present because the amount of carbon in an organic molecule varies between different organic materials. For example a sucrose molecule contains 12 carbon atoms, whereas a molecule of methanol contains only one carbon atom. Should a TOC analyser report 100 ppb TOC, it may mean that the water contains a large number of molecules of an organic material that has very few carbon atoms present, or it may be that there is a much smaller number of molecules containing a larger number of carbon atoms per molecule.
| Common terms describing carbon in water | |
| Total Organic Carbon | TOC |
| Total Carbon | TC |
| Total Inorganic Carbon | TIC |
Figure 1. Common terms describing carbon in water
Measurement Accuracy
All TOC analysers in common use on pharmaceutical water systems share the goal of oxidizing the organic material present in the water3 and then measuring the resultant carbon dioxide released from the oxidised organic molecule. Some analysers measure this carbon dioxide in gas phase, others measure in dissolved phase. Various methods are used to oxidise the organic with exposure to ultra-violet (UV) light, persulphate in the presence of UV light and high temperature combustion being the three most common types used in the pharmaceutical industry.
ICH Q2 discusses measurement accuracy and suggests accuracy may be inferred once precision, linearity and specificity have been established and suggests that linearity is established using a minimum of 5 concentrations of the traceable standard. The Joint Committee for Guides in Metrology suggest in their Guide to the expression of uncertainty in measurement4 that the higher the level of complexity in a measurement, the higher the measurement uncertainties due simply to the larger number of approximations and assumptions incorporated in the measurement method, thus impacting on the accuracy and the analyser’s ability to measure very low levels of analyte.
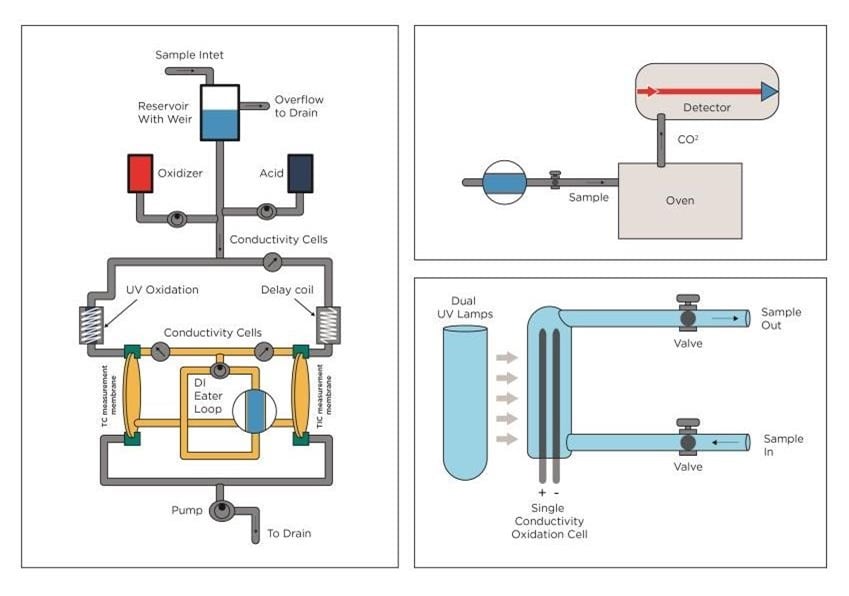
Figure 2. TOC technologies. From left: UV Persulphate combined with membrane conductometric, High Temperature Combustion and UV only.
Specificity Challenges
In their ICH Q2 guidance document Validation of Analytical Procedures2, the International Conference on Harmonisation highlights the need for an analytical procedure to have specificity, i.e. “the ability to assess unequivocally the analyte in the presence of components which may be expected to be present”3. One of the main specificity challenges to measuring the carbon dioxide from organic material in water is that pharmaceutical waters also contain relatively large amounts of total inorganic carbon (TIC) in the form of carbonates and dissolved carbon dioxide gas largely due to the increased concentration of dissolved carbon dioxide gas in the water caused by the reverse-osmosis process (RO) used to manufacture pharmaceutical water. So it can be challenging when trying to measure very low TOC water when there is a large amount of TIC present, especially in those TOC analysers that use multiple sensors to measure TC and TIC and then subtract one from the other to calculate the TOC content of the water (see Figure.3).TOC = TC - TIC
Figure 3. Calculating TOC from Total Carbon (TC) and Total Inorganic Carbon (TIC).
Analysers that rely on calculating the TOC from TC and TIC face a challenge when trying to measure very low amounts of TOC in the presence of relatively high amounts of TIC because relatively small inaccuracies between the TIC and TC sensors can lead to either over- or under-reporting of TOC5 (see Figure 4).
| Total Organic Carbon (TC) | 2,000 ppb |
| Total Inorganic Carbon (TIC) present | 1,900 ppb |
| Actual TOC present | 100 ppb |
| Analyser measurement accuracy | 2% |
| Analyser measured TC | Between 1,960 and 2,040 ppb |
| Analyser measured TC | Between 1,862 and 1,938 ppb |
| Analyser calculated TOC | Between 22 and 178 ppb |
Figure 4. Example showing that TOC analysers that use multiple sensors to measure TC and TIC and then calculate TOC can suffer from measurement inaccuracies5
It can be very difficult to be confident that the pharmaceutical water is of the correct quality for production when the inherent measurement uncertainties in the TOC analyser can lead to a potential inaccuracy in reported TOC level of +/-78% (based on the example in Figure 4).
The problem is compounded for quality control laboratories wishing to measure TOC in their incoming water supply. Seasonal variations in TIC levels will mean that the user has to invest in some sort of TIC removal device and constantly check on the levels of TIC in their incoming water to make sure that it never exceeds the maximum level recommended by their TOC analyser manufacturer. Some analyser manufacturers recommend a maximum ratio of TIC to TOC of 10:16, thus in a water sample containing 10 ppb TOC, the TIC must not exceed 100 ppb for that analyser to work correctly.
Typically, high temperature combustion analysers try to get around the problem of TIC by incorporating a TIC removal step. The water sample pH is shifted by the addition of an acid, forcing the TIC to come out of solution in the form of carbon dioxide gas. The carbon dioxide from the TIC is then sparged out of solution by bubbling a CO2-free carrier gas through the sample. These sparging cycles are typically of fixed duration and there is a danger that all of the TIC may not be removed and some may remain and interfere with the TOC analysis, so users again have to measure TIC levels in the water sample to ensure that they do not exceed the maximum levels specified by their TOC analyser manufacturer.
An alternative is to monitor the TIC removal to ensure that the TIC is completely removed before commencing TOC analysis. This method avoids the TIC specificity challenge and TOC measurement accuracy is independent to the levels of TIC present. The method can be further improved by using a single CO2 sensor to measure both the TIC and the TOC. Instead of calculating TOC from TC and TIC, this method directly measures the CO2 from the TOC in a separate measurement once all of the TIC is completely removed. The measurement sensor accuracy of +/- 2% now relates solely to the measured TOC value instead of the measured TC and TIC values used by the other methods. So using the example in Fig. 4 where the actual TOC value is 100 ppb, this method would report between 98 ppb and 102 ppb, giving the user far more confidence that the reported TOC results accurately reflect the actual amount of TOC in the water froma direct measurement, rather than a calculation.
This alternative method of course relies on the analyser being able to measure the complete removal of the TIC. The sensor must be able to measure when the CO2 from the TIC has been removed before the ultra violet light is turned on and oxidation of the organics to CO2 commences.
The Detection Limit Challenge
The ICH Q2 guidance document differentiates between three analytical procedures: Identification, Testing for Impurities and Assay. Although the document suggests that the quantitation limit of an analyser may not be relevant in an impurity limit test, such as the TOC test, it does state that detection limit is an important characteristic for such tests.
As mentioned earlier in this paper, TOC analysers report the weight in parts per billion (ppb) of carbon derived from organic material in the water. This brings its own challenge as modern pharmaceutical water systems may contain <10 ppb TOC and many laboratory TOC analysis technologies will struggle to report accurately at these low levels. Thus the analyser is not able to report the level of TOC and the user is left with error messages such as “TOC level is below the limit of detection”. Of course many users do not realise this because the act of taking a grab sample from a water system will unavoidably contaminate the sample leading to TOC readings typically excess of 100 ppb. So owners of very low TOC water systems may well be, in fact, measuring and reporting the TOC contamination from the grab sampling process, not the TOC in their water system.
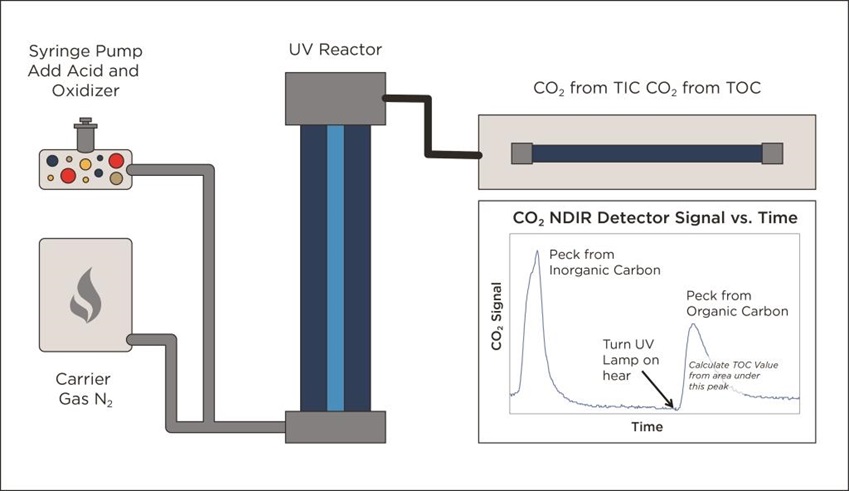
Figure 5. Alternative UV/Persulphate design monitors TIC removal before starting TOC analysis.
Very low TOC levels are even more challenging for analysers that employing multiple sensors and estimate TOC by subtracting measured TIC from TC. The analyser may actually report an estimated TOC value, even when the inherent accuracy errors in the multiple sensors used to measure the TC and TIC can have such a large impact in the accuracy of the reported TOC value5, as shown in Figure 4.
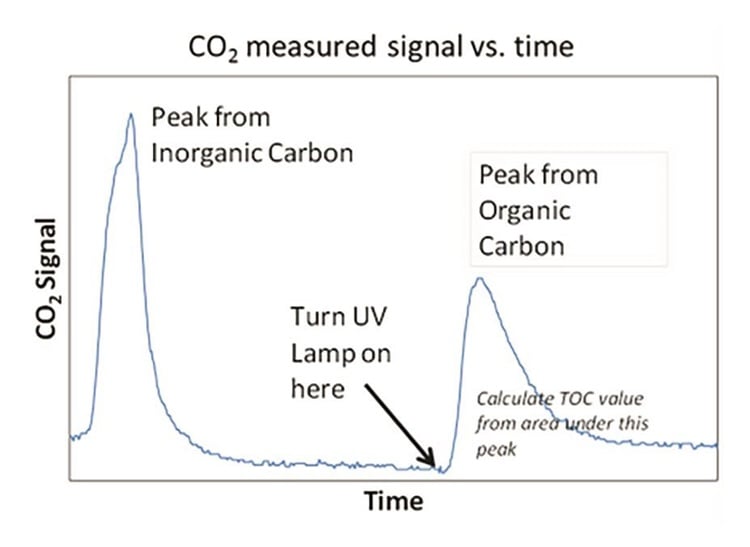
Figure 6. CO2 measured signal versus time
Whilst it is understood that some analysers cannot resolve down to these low levels of TOC accurately and will just report “TOC level is below the limit of detection”, it is uncomfortable to release a batch of product using an absence of data when it could be possible that the analyser actually failed to take a correct measurement because it ran out of carrier gas, or oxidising reagent. The user must make sure that the carrier gas and reagents are present before and after analysis and this is typically done by adding certified 500 ppb TOC standards into the batch of water samples to be analysed at the start, middle and end of the analyser’s autosampler tray. However, as laboratory TOC analysers are frequently set up and used overnight, a failure in carrier gas, or reagent supply during the night can mean that the user is aware that the results from the batch of water samples is not correct when they check the analyser the next day, but then cannot re-test the batch of water samples because the analyser has used all the samples up trying to analyse them during the night. This can leave the user with no proof that the water system was in compliance during the batch of product manufactured the day before.
Conclusion
Accurate total organic carbon analysis of low TOC modern pharmaceutical grade water faces many challenges. Instruments using multiple sensors to measure TC and TIC and then calculate the TOC can suffer from inaccurate results due to TC and TIC measurement inaccuracies5. Analysers using just one sensor to make the measurements can deliver a more accurate result because there are fewer approximations and assumptions in the measurement4.
Specificity in the presence of inorganic carbon is a challenge for many analysers. A more accurate method is to remove the TIC and to monitor that it has been removed completely before measuring the TOC directly.
Many analyser designs are simply unable to measure low ppb TOC levels due to the analysers’ limit of detection due to the multiple approximations and assumptions in the measurement4. Although the pharmacopoeias require the TOC analyser to have a limit of detection of 50 ppb1, this is just not sufficient when measuring water from a modern, low ppb TOC system.
Users wishing to use a wider ranging TOC analyser that uses a combination of oxidising reagents and/or carrier gases should put in place methods to ensure that the analyser cannot continue to carry out analyses and destroy water samples should either the reagent or the gas run out. This can be a manual check, or can be designed into the analyser so that it continuously monitors all of the critical analysis parameters and stops trying to carry out analyses should anything go wrong.
The guidance given in the ICH Q22 guidance document can help users to determine the suitability of the design and performance of laboratory TOC analysers in the light of the challenges of measuring TOC in modern low-TOC water systems described in this paper.
References
- US Pharmacopeia Convention, United States Pharmacopoeia, Rockville MD, USA and Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia, Strasbourg, France
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Validation Of Analytical Procedures: Text And Methodology Q2(R1), November 2005 [8th August 2014], http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf [8th August 2014]
- Council of Europe, European Directorate for the Quality of Medicines & Healthcare, European Pharmacopoeia 8.0, 01/2008:20244, Total Organic Carbon in Water for Pharmaceutical Use, Strasbourg, France
- Joint Committee for Guides in Metrology, Evaluation of measurement data — Guide to the expression of uncertainty in measurement ref. JCGM 100:2008 First edition September 2008, http://www.iso.org/sites/JCGM/GUM-JCGM100.htm [8th august 2014]
- GE Analytical Instruments, Technical Bulletin The Sievers Inorganic Carbon Remover (ICR), Boulder, Colorado, USA www.geinstruments.com [8th August 2014]
- GE Analytical Instruments, Sievers 900 Series Total Organic Carbon Analyzers, Operation and Maintenance Manual, Ref. DLM 90688-03 Rev. A, 2011, Boulder, Colorado, USA www.geinstruments.com [8th August 2014]
- International Society for Pharmaceutical Engineering, The ISPE Good Practice Guide: Ozone Sanitization of Pharmaceutical Water Systems, First edition July 2012 http://www.ispe.org/ispe-good-practice-guides/ozone-sanitization-pharmaceutical-water-systems [14th August 2014]
- Pharmaceutical and Healthcare Sciences Society, Best Practice for Particle Monitoring in Pharmaceutical Facilities, PHSS Technical Monograph No.16, First Edition 2008, ISBN 978-1-905271-15-3
About the Author
Tony Harrison is a Compliance and Applications Specialist for Beckman Coulter Life Sciences. An experienced engineer in water system TOC, pH, conductivity and ozone analysis, Tony has spent the last twelve years in applied metrology in the pharmaceutical and healthcare manufacturing industries. Prior to that, he worked for companies providing process control automation solutions for manufacturing industries.
Tony was joint-editor of the ISPE Guide to Ozone Sanitization of Pharmaceutical Water Systems7 and was also chief editor of the PHSS Best Practice Guide for Cleanroom Monitoring8.
Tony is a well-known international speaker and has provided educational seminars on TOC, liquid particle counting, ozone sanitization for water systems and cleanroom monitoring in UK, France, Italy, India, Germany, Malaysia, China, USA, Scandinavia, Ireland, Hungary, Switzerland, Indonesia, Belgium, Greece, Switzerland, Turkey, Egypt and Denmark.


