Centrifugation is a complete workflow solution for protein purification and protein aggregation quantification
Content Type: White Paper
Julia P Luciano-Chadee | Beckman Coulter, Inc., Indianapolis, IN 46268
Many factors can influence protein behavior and aggregation, including temperature, oxidation, concentration, pH, ionic strength, additives, detergent, cryoprotectants, freeze-thaw cycles, and storage and handling conditions.
Centrifugation is a powerful tool for workflows to isolate and characterize protein products.4 The Beckman Coulter Life Sciences centrifuge portfolio delivers quality separations and state-of-the-art protein analysis, offering users comprehensive solutions from beginning to end. A centrifugation-based workflow solution can be highly effective because it systematically addresses the need to remove aggregates by employing increasing g-force and density gradients to separate particles based on mass and density.
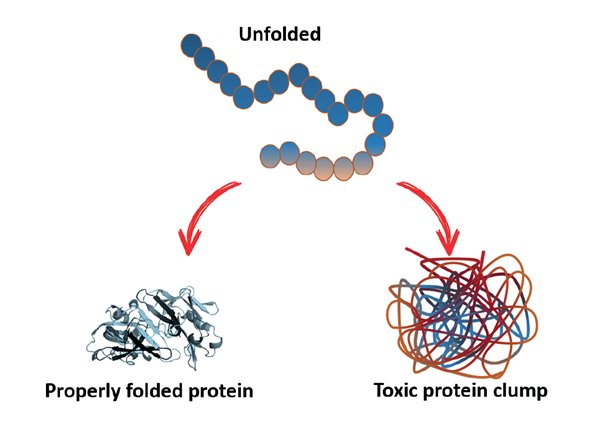 |
| An unfolded polypeptide chain collapses into a properly folded three-dimensional protein structure. Conversely, if the key interactions are not formed, the unstable protein can aggregate and form toxic protein clumps |
 |
| Avanti high-speed centrifuges |
Centrifugation of crude protein mixtures is a required step in the study of protein purification and aggregation
Upstream of the study of protein aggregates is protein purification.5 Typically, proteins are overexpressed in an organism, like bacteria, yeast, or mammalian cells in culture. Characteristics unique to each protein, such as amino acid composition, size, shape, isoelectric point, and solubility are used to develop unique strategies for isolating the protein of interest. The objective is to isolate the largest amount of a functional protein of interest with the least amount of other contaminants. Centrifugation is an important, and often first step, in any protein purification protocol. The Avanti JXN Series of high-speed centrifuges has an array of rotors available to help with any stage of protein purification. This extensive selection of rotors--with different combinations of volume and g-force--makes the JXN a versatile instrument capable of meeting the needs of a wide array of proteomic projects, and it is amenable to different buffers to promote protein stability. Centrifugal protein purification with a highperformance Avanti JXN series is robust, and it provides users with a highly reproducible technique.
 |
| Optima XPN-100 Ultracentrifuge |
 |
| Optima AUC analytical ultracentrifuge |
Ultracentrifugation is a powerful step to separate proteins from aggregates based on size
It is well established that centrifugation is a powerful and generally applicable method for separating a crude mixture of cell components.4,5 Furthermore, ultracentrifugation combined with density gradient is a major technique for separating and analyzing proteins of interest based on size and mass.6 During a centrifugal spin under a specific gravitational force, molecules move from the top to the bottom of the solution in a tube. This movement is offset by the density and viscosity of the liquid, or the buoyance force. At equilibrium, the net effect of the gravitational and buoyant forces will lead to accumulation of specific proteins at a specific point in the tube, thereby separating proteins from aggregates with different sedimentation coefficients.6 Denser aggregated proteins move outward faster than lighter individual protein molecules.
Continuous rate-zonal density gradients are formed by first layering a step gradient. In the most common technique, an aliquot of a less dense solution is first pipetted into a centrifuge tube and successively denser solutions are introduced to the bottom of the tube using a long syringe so as to not disturb the previous layer, leaving a sharp interface between the different density layers. An alternative approach layers decreasingly dense solutions gently on top of denser solutions. A continuous gradient can also be generated from a discontinuous gradient by incubating or spinning the solution for a pre-set period of time, or by using a commercial gradient maker. A third method, buoyant density gradient, involves prolonged high speed centrifugation of a molecule at similar solution density, resulting in stringent molecule separation.
In all these techniques, the solution diffuses such that a gradual increase in density is produced from the top to the bottom of the tube.
Subsequent to the gradient formation, a small volume of a solution containing the protein mixture is placed on top of the density gradient. Almost always, when the rotor is spun, proteins move through the gradient and separate according to their sedimentation coefficients, with protein aggregates sedimenting faster than an individual protein product of interest. The time and speed of centrifugation is determined empirically.
After centrifugation, the separated proteins are typically harvested by puncturing a hole at the bottom of the tube and collecting fractions dropwise, or by manually pipetting the fractions from the meniscus. Biochemical or functional assays can be performed to assess the protein content of each fraction.
In addition to protein aggregation separation, purification of protein-ligand complex is often used in the study of protein-based therapeutics. The Optima XPN Ultracentrifuge series includes preparative centrifuges compatible with an array of rotors and consumables suitable for a variety of protocols in the protein purification workflow.
A commonly used technique in protein separation is size exclusion chromatography (SEC).4 When compared to centrifugation, SEC requires a large dilution factor, protein incompatibilities and undesirable interactions between proteins and resin (matrix effect), and low resolution. Density gradient centrifugation is advantageous because it enables users to adjust parameters for efficient separations governed by the laws of thermodynamics, amendable to all proteins following spin time, speed and gradient condition optimization.7
Oxidative exposure of proteins at the airliquid interface can also result in an increase in aggregation.1,4,5 High amounts of reducing agent are necessary in buffer solutions for SEC separation, which can add to the cost of an experiment. Furthermore, the typical buffer solution is stored in high-volume media bottles, with increasing air-liquid surface area. Hermetic sealing of the Opti-Seal and Quick-Seal tubes compatible with Beckman Coulter ultracentrifuges minimizes the air-liquid interface, leading to a more stable protein product and reduced aggregation. Moreover, centrifugation circumvents the need to use fusion tags, a common downfall of chromatography-based protein purification techniques,8 which can drastically impact protein solubility.
Analytical Ultracentrifugation is a powerful tool for analyzing proteins from aggregates
As discussed above, SEC as a separation tool has major flaws, including significant dilution and matrix effects. Therefore it is not ideal for critical assessment of particle size and aggregation of protein-based products. Moreover, it relies on standards to assess molecular weight, which introduces an extra variable that increases the margin of error and potential unreliability of the SEC method.9
In contrast, Analytical Ultracentrifugation (AUC) analysis provides users with more answers than any comparable technique. AUC is the most accurate and powerful tool10 to quantify protein aggregation. It is an absolute method that relies on the first principle physics model to determine molecular shape, weight and complex formation at high resolution using a range of sample sizes and undiluted concentrations. Further, the standard-free and matrix-free minimal surface interaction gives users the ability to use a variety of possible buffer compositions.
In the study of protein products and aggregation, buffer composition (pH, ionic strength, additives, etc.) is of most importance, as it directly influences protein stability.7,8,9,10 AUC aids in lead drug-candidate selection, biotherapeutic formulation development, product characterization and stability assessments.11 Use of AUC in product quality analysis by biopharmaceutical companies can help effectively identify possible adverse immune response of protein-based drugs or biosimilars. The new Optima AUC expands the scope of solutions provided by AUC, and provides users with well-determined macromolecular sedimentation parameters and aggregation characterization.
References
- Philo JS, Arakawa T. Mechanisms of protein aggregation. Current Pharma Biotech 2009;10:348-51.
- Rosenberg AS. Effects of protein aggregates: An immunologic perspective. AAPS Journ 2006;8(3):E501-07.
- Niazi SK. Biosimilars and Interchangeable Biologics: Tactical Elements. Boca Raton (FL): CRC Press; 2016.
- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. Section 3.5, Purifying, Detecting, and Characterizing Proteins. 4th ed. New York (NY): W.H. Freeman; 2000.
- Berg JM, Tymoczko JL, Stryer L. Biochemistry. Section 4.1, The Purification of Proteins Is an Essential First Step in Understanding Their Function. 5th ed. New York (NY): W.H. Freeman; 2002.
- Graham JM. Biological Centrifugation. 1st ed. Oxford (UK): Bios Scientific Publishers Ltd; 2001.
- Berkowitz, SA. Role of analytical ultracentrifugation in assessing the aggregation of protein biopharmaceuticals. AAPS Journ 2006;8(3):E590-E605.
- Louis JM, McDonald RA, Nashed NT, et al. Autoprocessing of the HIV-1 protease using purified wild-type and mutated fusion proteins expressed at high levels in Escherichia coli. Eur J Biochem 1991;199:361–69.
- Gabrielson JP, Arthur KK, Stoner MR, et al. Precision of protein aggregation measurements by sedimentation velocity analytical ultracentrifugation in biopharmaceutical applications. Anal Biochem 2009;396:231–41.
- Arthur KK, Kendrick BS, Gabrielson JP. Chapter Twenty – Guidance to achieve accurate aggregate quantitation in biopharmaceuticals by SV-AUC. Methods Enzym 2015;562:477-500.
- Pekar A, Sukumar M. Quantitation of aggregates in therapeutic proteins using sedimentation velocity analytical ultracentrifugation: practical considerations that affect precision and accuracy. Analyt Biochem 2007;367(2):225-37.
CENT-2269WP01.17

