A High-Throughput, Automated Screening Platform for IgG Quantification During Drug Discovery and Development
Dr. Paul Sweeney and Dr. Hannah Byrne • ValitaCell (now a part of Beckman Coulter Life Sciences)Introduction
The global biologics market was estimated worth over $450 billion in 2022, with an annual growth rate of 10% to 20301. Amongst these biological products are monoclonal antibodies (mAbs), which dominate the biologics landscape with over 50% of that market share1. One of the most critical stages in the development of new mAbs is cell line development (CLD). In a CLD process, thousands of individual cell clones will be screened to find the best and most stable producer of a target therapeutic. This screening process is laborious, cost-intensive and time-consuming, whereby most of the screening requires a significant amount of hands-on time and manual input from CLD scientists. One of the most important screening and monitoring parameters performed routinely throughout CLD is to quantify the amount of target therapeutic being produced by each individual clone. Quantification of mAbs (e.g., IgG) is vital to identify the best candidate for large-scale production.
Commonly used methods to quantify mAbs can include HPLC or surface interferometry, which rely on the use of special instrumentation with an associated high CAPEX investment and highly skilled analysts. In addition, these techniques are typically challenging to fully automate, limiting their use for high-throughput, walk-away screening. Other routes to quantitate mAbs can be very hands-on and time-consuming (e.g., ELISA). Another common obstacle which scientists face when performing mAb quantitation is that a lot of techniques require sample pre-preparation or cleanup steps to remove cells or other matrix components.
The Valita Titer products are rapid, high-throughput and fully automatable plate-based (96- or 384- well) assays that facilitate the quantification of IgG from 2.5 mg/L – 2000 mg/L in the presence of cells.
Here, we present the Valita Titer assay combined with a Biomek i5 liquid handling system and Molecular Devices SpectraMax® iD5 Multi-Mode Microplate Reader (Figure 1) which provide a high-throughput fully automated and reproducible solution for accurate IgG quantification throughout drug development and manufacturing. The solution presented here demonstrates the quantification of up to 456 samples (5 x 96-well plates) in a little over an hour with the presentation of a direct output of IgG titer results. Two other experiments were performed as part of this work to 1) demonstrate the ability of this workflow to accurately quantitate IgG in the presence of Chinese Hamster Ovary (CHO) cells, and 2) demonstrate the utilization of dilution factors to expand the assay range simply by inputting desired acquisition settings to the Biomek CLD protocol.

Figure 1. The technologies used in this work to acquire high-throughput IgG quantification. 1) The Valita Titer IgG quantification assay plate; 2) The Biomek i5 liquid handler, responsible for standard curve creation and plating of standards and samples; 3) Molecular Devices SpectraMax iD5 reader, which performed FP measurement on the Valita Titer assay plate
Principle
The Valita Titer and Valita Titer Plus assays are rapid detection assays that quantitate all Fc-containing IgGs using a technology called fluorescence polarization (FP). Each well of the assay plates comes pre-coated with a fluorescently labelled protein G derivative which is reconstituted prior to test sample addition. This fluorescent molecule when in solution will rotate rapidly, producing a non-polarized emission of light after excitation with plane polarized light. When the fluorophore is attached to a larger molecule (i.e., bound to the Fc of an IgG), the rotation will slow, resulting in the emission of polarized light (Figure 2). The measurement of the degree of light polarization allows us to calculate a proportional amount of Fc-specific probe attached to its IgG target.
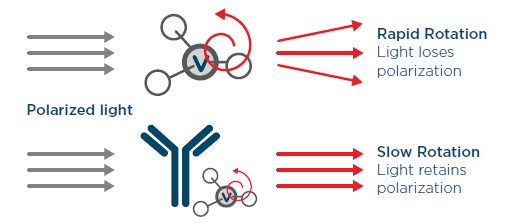
Figure 2. The fluorescence polarization principle used in the Valita Titer assay. Small, unbound fluorescently labeled proteins rotate rapidly in solution, emitting less polarized light (top), while bound fluorescently labeled proteins, as part of a larger molecular complex rotate slowly, emitting increased polarized light (bottom).
The Biomek i5 liquid handler with 96-channel head is responsible for all pipetting and mixing steps presented in this application note. The multichannel head allows for liquid transfers and mixing using either single tip format for varied aliquoting (e.g., standard curve preparation) or up to 96 tips for bulk solution transfers (e.g., probe resuspension). The user only needs to add the 1 mg/mL IgG standard to the deck, along with sample plates, test plates, pipette tips, and buffer. This results in the preparation of 1 to 5 96-well test plates on the deck with user input to the Biomek software dictating how many samples need assessment (from 1 to 456 samples) and what dilution factor these samples are required to undergo (from 1- to 6-fold dilutions).
The SpectraMax iD5 reader performed all analytical measurements required for this screening platform. The SpectraMax iD5 reader can perform fluorescence polarization (FP) allowing measurement of Valita Titer plates with automated interpolation of results for each sample analyzed against the assay-included standard curve. This results in the precise and accurate presentation of results directly after each plate read.
Materials
Experiments 1) 456-Sample Assay and 2) 3-Fold Dilution Factor Assay
- Valita Titer Rapid High-Throughput IgG Quantification Assay 96-Well (ValitaCell/Beckman CoulterP/N VAL003)
- Biomek i5 Multichannel Liquid Handler – OR Biomek i7 Multichannel Liquid Handler (with 96-Multichannel, Beckman Coulter)
- Biomek Consumables
- Biomek i-Series Tips (Beckman Coulter, Non-Sterile)
- 80 μL P/N B85764
- 230 μL P/N B85903
- 1070 μL P/N B85940
- 96-Well Microplate, Flat, Non-Sterile (Beckman Coulter P/N 609844)
- 24 Position Microfuge Tube Rack (Beckman Coulter P/N 373661)
- Flip Cap Insert, 1.5 mL for Microfuge Rack (Beckman Coulter P/N 373696)
- Biomek i-Series Tips (Beckman Coulter, Non-Sterile)
- Black Universal Microplate Lids (Corning P/N 10565672)
- Low Protein Binding Microcentrifuge Tubes (Thermo Scientific P/N 90410)
- CD CHO Medium (Thermo Fisher Scientific P/N 10743-011)
- IgG1k Drug Product
- SpectraMax iD5 reader equipped with 485 nm FP excitation and 535 nm FP emission filters (Molecular Devices P/N 6590-0136 and 6590-0137)
- SoftMax Pro Software (Molecular Devices)
- GraphPad Prism 10 (used here for data presentation, SoftMax Pro Software can also handle all required analysis and standard curve generation)
Experiment 3) Titer Assay in Presence of CHO Cells
This assay used the same materials as the other two assays but with the following usage for media and cells:
- BalanCD CHO Growth A Medium (Fujifilm P/N 911281L)
- K1 CHO cells
- Countess 3 FL (Invitrogen)
Workflow (Experiment 1 - Fully Automated Quantification of 456 Test Samples across 5 x 96-well plates)
Preparation of test plates
A master stock of IgG was diluted to a 1 mg/mL concentration. This was used to produce solutions at 10, 20, 30, 40, 45, 50, 65, and 80 mg/L in fresh CD-CHO medium to mimic a range of screening samples. The samples (200 μL) were added to 96-well plates, administered to all wells not designated for test standards (Figure 3). 58 replicates of each concentration were prepared for analysis with the exception of the 10 and 20 mg/L samples (n = 54) where a single well for each concentration was substituted with a high and low control for each plate (excluding plate 1).

Figure 3. Plate layout for sample addition to Biomek deck. Sample is added to all wells except for those indicated by red boxes which will be administered standards by the Biomek liquid handler. Plate 1 (left) leaves columns 1 and 2 empty for an 8-point standard curve in duplicate. Plate 2 and all subsequent test plates (right) leave wells A1 and B1 empty for ‘high’ and ‘low’ control additions
Set up and performance of Biomek i-Series Liquid Handler
Biomek method launcher was opened, and Titer CLD Screen protocol was selected to run (Figure 4). This brings up a request for the number of samples under assessment in this assay as well as the dilution factor that the user expects to need. 456 samples and a dilution factor of 1 were selected.

Figure 4. Biomek method launcher interface. Method launcher will present the user with available methods for selection (in this case the Titer CLD Screen, left), followed by a request for the number of samples and dilution factor required for the assay (right)
A total of 5 Valita Titer plates were stacked on the deck as prompted by the Biomek software, along with 1 mL of the 1 mg/L working stock of IgG standard. CD-CHO media (100 mL) was added to a reservoir on deck. All full and empty tip boxes were added to the deck as directed by the instrument setup in the Biomek software (Figure 5). This deck layout will change depending on how many samples are being submitted for analysis. The sample and test plates are stacked at the front of the deck, the Titer plates topped with a dark lid to prevent any unnecessary light exposure to fluorophores contained within the plate wells.

Figure 5. Biomek i5 deck layout for analyzing up to 5 x 96-well test sample plates. Automated lab positioners (ALPs) are labelled as in the left image. Note tip loading (TL) positions 1-5 and their orientation. The position of all consumables, including stacked test and sample plates is demonstrated in the right image
The software was prompted to ‘Start run’, which initiated the automated preparation of the standard curve from the 1 mg/mL working stock standard and CD-CHO media. Following this, the liquid handling unit delivered the first sample plate to the P15 position and administered the standard curve in duplicate to columns 1 and 2 of the 96-well plate. The gripper then delivered the first Valita Titer plate to the deck and the multichannel head dispensed 60 μL of CD-CHO media to each well to resuspend the probe. Immediately following this the multichannel head aspirated 60 μL of standard and sample from all wells of the sample plate to the Valita Titer test plate, followed by pipette mixing. The lid was then added to the test plate and this along with the sample plate were cycled back into their original plate stacks before this process was repeated. Instead of the application of standard curves to sample plates 2-5, a high and low standard were added to wells A1 and B1 respectively to examine plate performance and variability. The full automated preparation of these plates was complete in < 50 minutes.
Plate read using fluorescence polarization
The plates were each transferred to the SpectraMax iD5 reader and measured using FP with the parameters outlined in Table 1. Each plate was run with each following plate read performed immediately after the preceding plate. SoftMax Pro software presented the interpolated results of the 456 samples against a 5-parameter logistical (5-PL) standard curve derived from the standards in plate 1. The total assay time was 80 minutes with a breakdown of the assay timeline summarized in Figure 6).

Table 1. Instrument settings used on the Molecular Devices SpectraMax iD5

Figure 6. Timeline for the preparation and analysis of 5 x 96-well Valita Titer assay plates on the Biomek i-Series liquid handler with SpectraMax iD5 reader performing the detection using FP. Total assay time is reduced with a reduction in number of plates assayed.
*An extra 4 minutes total time can be added if using dilution factors and partial plate assays
Supplementary Workflows
Experiment 2 - Investigating the use of a 3-Fold Dilution Factor for the quantification of samples that fall outside the Valita Titer assay range
The assay was performed as with the 456-sample assay, this time with a reduced sample number (174 samples across two plates) and a dilution factor of 3. Sample concentrations of 30, 60, 90, 120, 150, 180, 210 and 240 mg/L were prepared (n=21 for 30 and 60 mg/L, n=22 for the remainder). The Biomek i-Series liquid handler took 34 minutes to prepare the assay plates. These Valita Titer plates were incubated at room temperature for 5 minutes after conclusion of the automated plate preparation.
Experiment 3 - Investigating the performance of Valita Titer assay for quantifying IgG in the presence of CHO Cells
This assay was performed as with the 456-sample assay, however a concentration of 5 x 10^6 CHO K1 cells per mL was present in each test sample and a total of 80 samples tested. A fresh passage of CHO K1 cells were incubated at 37 °C for 5 days. A viable cell population was measured using the Countess III FL. A concentration of 5 million cells per mL in each sample was achieved by contributing 1.5 mL of a 7.5 million cells per mL culture to a total IgG sample volume of 2.25 mL. Sample concentrations of 10, 20, 30, 40, 45, 50, 65 and 80 mg/L were analyzed (n = 10). The Biomek i-Series liquid handler completed the liquid handling segment of the assay in 23 minutes. This Valita Titer plate was incubated for 10 minutes after the completion of the sample administration by the Biomek i5 liquid handler.
Results
The IgG titer recovered for every sample in all assays performed was generated using SoftMax Pro software, including dilution factor back calculations. The coefficient of variation and standard deviations of the standards as well as the R2 for the 5-PL standard curves was automatically generated. The data provided by the software was extracted for further processing using Microsoft Excel to determine, for the goals of this work, the accuracy and variability displayed by combining the Valita Titer assay, Biomek i-Series liquid handling system and SpectraMax iD5 reader technologies.
Experiment 1 – Fully Automated Quantification of 456 Test Samples across 5 x 96-well plates
Standard Curve
The standard curve (Figure 7) was automatically generated using a 5-PL fit by the SoftMax Pro software. The duplicate standards produced a maximum % relative standard deviation (RSD) of 3.3 % (Table 2). The standard curve generated from this curve had an R2 of 0.999.

Table 2. Raw FP values and associated variability between duplicates for the standards in the 456-sample assay

Figure 7. Standard Curve for the 456-sample assay generated with SoftMax Pro software. The x-axis displays each standard concentration in mg/L. The y-axis displays the raw FP values for each data point. Error bars represent the RSD
Test Sample Titer Results (5 Sequential Plate Reads)
The 8 various concentrations analyzed across 5 plates were automatically interpolated against the standard curve using the SoftMax Pro software. All samples were plotted on a box chart using GraphPad Prism software (Figure 8). One sample point was omitted out of the 456 samples due to an outlying recovery of > 4-fold standard deviations from the mean for that sample subset. The values used to plot this data are summarized in Table 3. The average of all these sample sets recovered within 5% of their starting concentrations and within 5% RSD. The exception here is the 10 mg/L sample subset which may be disproportionately affected by any inherent deviations or variability that result from the manual and automated aspects of the workflow.
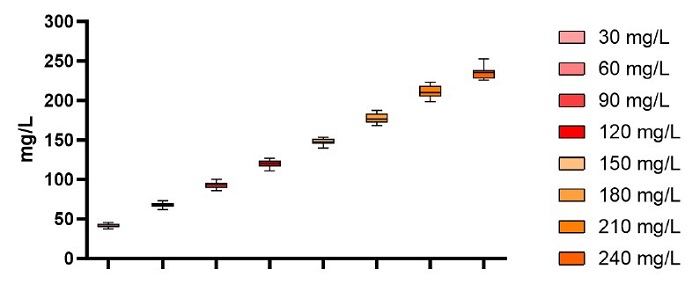
Figure 8. Box and whisker plot representing the IgG samples at different concentrations analyzed across the 5 Valita Titer assays plates

Table 3. Sample recoveries and variability of the 456-sample assay

Table 4. Sample recoveries of each individual plate of the 5-Valita Titer plate assay
Experiment 2: Investigating the use of a 3-Fold Dilution Factor for the quantification of samples that fall outside the Valita Titer assay range
Standard Curve
The standard curve run for this assay, in differentiation to the test samples was applied neat. These standards produced standard deviations of < 3 mP units in all cases and RSD values of < 2.5% (Table 5). The R2 for the 5-PL fit (Figure 9) was 0.997.
Table 5. Raw FP values and associated variability between duplicates for the standards in the 3-fold dilution factor assay

Figure 9. SoftMax Pro software-generated Standard curve for the 3-dilution factor assay. The x-axis displays each standard
concentration in mg/L. The y-axis displays the raw FP values for each data point. Error bars represent the RSD
Two 96-well plates or 174 test samples were analyzed in this assay. Each sample was diluted 3-fold on the Biomek i-Series liquid handler deck with the resultant Valita Titer assay IgG quantitation back calculated by the utilized dilution factor using SoftMax Pro software. This assay sought to investigate the accuracy and precision that could be yielded through using the on-deck sample dilution option, particularly as any deviations demonstrated within the assay would be expanded and exposed when multiplied by the dilution factor to yield the final results.
The quantitated titers of each sample set are illustrated in Figure 10. The accuracies and precision are tabulated in Table 6. As expected, due to the multiplication of titers to determine the original concentrations of each sample, the standard deviations have increased to 7 mP units. However, the RSDs are within a respectable 5.5%. The lowest point on the curve, once again demonstrated a higher recovery than anticipated. Despite this, the standard deviations exhibited provide no overlap of any of the 174 samples from one concentration subset with another sample that had a different starting concentration.

Figure 10. Box and whisker plot representing the IgG samples at different concentrations analyzed across the 2 Valita Titer assay plates after back calculating data to original sample concentrations

Table 6. Sample recoveries and variability of the 3-fold dilution factor assay
Experiment 3: Investigating the performance of Valita Titer assay for quantifying IgG in the presence of CHO Cells
Standard Curve
The standard curve run for this assay in contrary to the test samples was prepared by the Biomek i5 liquid handling system and applied to the Valita Titer plate without the presence of any cells. The duplicate standards observed a standard deviation of < 2 mP and < 2% RSD in all cases (Table 7). The R2 for the standard curve generated from these data points was 0.998 (Figure 11).

Table 7. Raw FP values and associated variability between duplicates for the standards in the CHO cell assay

Figure 11. SoftMax Pro software-generated Standard curve for the CHO cell administered assay. The x-axis displays each standard concentration in mg/L. The y-axis displays the raw FP values for each data point. Error bars represent the RSD
A single plate containing 80 samples in the presence of 5 x 10^6 CHO cells per milliliter was analyzed in this assay to (1) demonstrate the functionality of the Biomek i-Series tips to handle these cells; (2) assess the FP functionality of the SpectraMax iD5 reader to measure a test sample containing cells and (3) demonstrate the performance of the Valita Titer assay to measure IgG titer accurately in the presence of cells. The individual IgG recoveries of the 80 samples determined from the Titer protocol are all displayed in Figure 12. No sample subsets, including the three tightly spaced 40 to 50 mg/L groupings demonstrated an overlap in results. All RSD values are less than 4% for the 10 replicates of each sample type (Table 8).

Figure 12. Scatter plot of the recovered concentrations of all 80 CHO cell suspended samples tested in a single Valita Titer plate

Table 8. Sample recoveries and variability of the Titer assay performed in the presence of CHO cells
Discussion
The assays performed in this work outline the functionality of a workflow that provides automated assay preparation, readily interpolated IgG content acquisition with precision and accuracy in delivery of these results. The IgG quantification was provided using variables such as the presence of CHO cells, dilution factors and with sequential running of plates. In the case of single plate runs, a short incubation time is necessary (from 5 minutes) before plate reads. With each plate taking 5 minutes to prepare on deck, multiple plates can be analyzed one after another with high precision between plates as verified in this work. In the vast majority of sample sets analyzed in these assays, the accuracy was no more than a few percent from their absolute recoveries. In some instances, lower assay concentrations trended higher than anticipated. However, these low concentrated sample sets did not overlap with their neighboring sample concentration subsets illustrating the precision between points and reliability in differentiating IgG production from differing clones. This is further exemplified in experiment 3, where samples of 40, 45 and 50 mg/L provided no overlap in interpolated recoveries from the 10 replicates provided by each sample set despite the presence of CHO cells.
This workflow allows the user to analyze any number of samples, up to 456 in a single assay with integrated dilutions performed to assist measurements of clones with differing IgG output. The user-friendly options to this protocol on the Biomek software combined with the simple deck set-up allow for quick turnaround between multiple assay occasions if necessary. Hourly performance of this assay in full capacity can allow for thousands of CLD samples to be analyzed each day. This workflow provides an extra step towards higher throughput analysis of cell lines, reliably and accurately measuring for antibody titer.
References
1 - https://www.grandviewresearch.com/industry-analysis/biologics-market


