Biomek i-Series Automated Beckman Coulter Agencourt RNAdvance Blood Kit
Biomek Automated Genomic Sample Prep Accelerates ResearchIntroduction
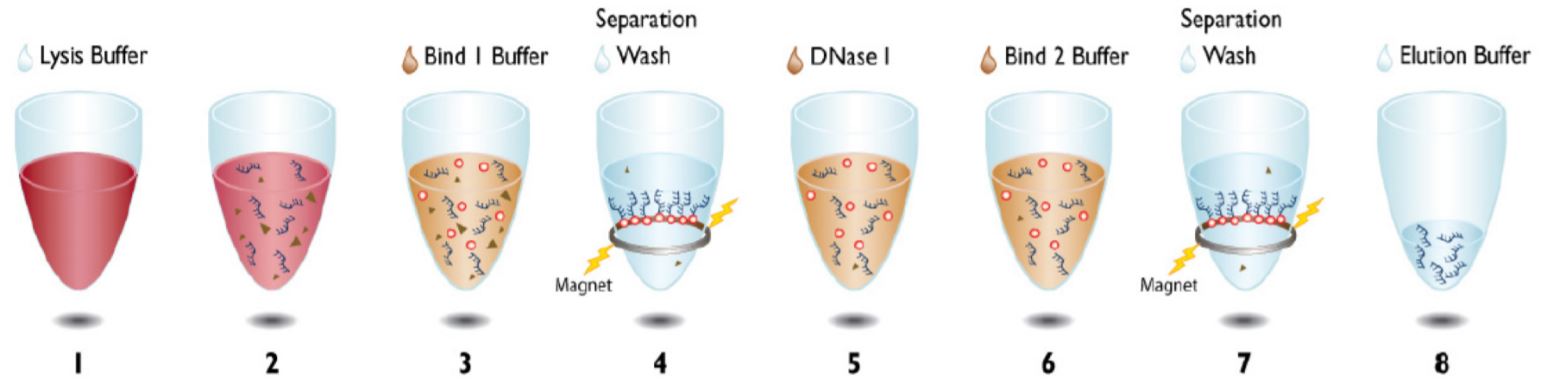
The Agencourt RNAdvance Blood Kit uses Beckman Coulter’s patented Agencourt SPRI paramagnetic bead-based technology to isolate total RNA (microRNA and mRNA) from PAXgene preserved blood. The protocol can be performed in both 96-well and single tube formats. Purification begins with lysis and protein digestion. Following lysis, the RNA is immobilized onto the magnetic particles allowing separation from contaminants using a magnetic field (Figure 1). The RNA is then treated with DNase and the contaminants rinsed away using a simple wash procedure. The Agencourt RNAdvance Blood kit is amenable to automation as it utilizes magnetic separation, thus eliminating the need for vacuum filtration or centrifugation.
Here we demonstrate the performance of Agencourt RNAdvance Blood Kit on the automated Biomek i7 Hybrid Genomics Workstation.
When compared to manual operation, the Agencourt RNAdvance Blood Kit automated on Biomek platform provides:
- Reduced hands-on-time and increased throughput
- Reduction in pipetting errors
- Standardized workflow for improved results
- Quick implementation with ready-to-implement methods
- Knowledgeable support for reagents, automation and methods all from single vendor
|
Spotlight: Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation System features deliver reliability and efficiency to increase user confidence and walk-away time.
|
Biomek i7 Hybrid Genomics Workstation with optional enclosure on a Biomek Cart. Large Deck capacity to increase walk away time. |
Agencourt® RNAdvance™ Blood
Figure 1. Beckman Coulter Agencourt RNAdvance Blood Kit protocol.
| Major Process Description | Time | ||
| 24 Samples | 48 Samples | 96 Samples | |
| Prepare Reagents/ Instrument Set up | 15 min | 15 min | 15 min |
| Method Run | 2 hrs 10 min | 2 hrs 16 min | 2 hrs 47 min |
| Total | 2 hrs 25 min | 2 hrs 31 min | 3 hrs 2 min |
Timing does not include thawing of reagents
Lysis incubation is off deck = 15 mins
Table 1. Estimated run times for Agencourt RNAdvance Blood Kit on the Biomek i7 Hybrid Genomics Workstation.
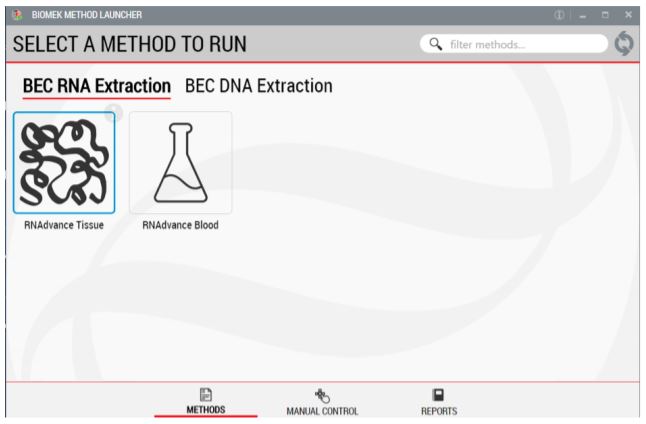
Figure 2. Biomek Method Launcher provided an easy interface to start the method
Figure 3. Biomek Method Options Selector indicates sample number and processing options
Automated Method
Automation provides increased efficiency, reducing the hands-on time (Table 1). The method also enables blood transfer from PAXgene tubes to a 96 well plate on the deck. It also uses enhanced selective tip pipetting feature on the multichannel head to do partial plates. The use of Biomek Method Launcher simplifies the method implementation and reduces the introduction of errors during method setup.
1. Biomek Method Launcher (BML)
BML is a secure interface for selecting methods without affecting method integrity. It allows the users to remotely monitor the progress of the run. The manual control options provide the opportunity to interact with the instrument, if needed.
2. Method Options Selector (MOS)
MOS enables selection of plate processing and sample number options to maximize flexibility, adaptability and the ease of method execution.
3. Guided Labware Setup (GLS)
GLS is generated based on options selected in the MOS, and provides the user specific text and graphical setup instructions with reagent volume calculation and step by step instructions to prepare reagents.
Figure 4. Guided Labware Setup indicates reagent volumes and guides the user for correct deck setup
Experimental Design
Four technical replicates of 400uL of blood were used to extract RNA using both manual and automated Agencourt RNAdvance Blood kit protocols. The quantity and the quality of the RNA samples were assessed using NanoDrop 2000 TM (Thermo Fisher Scientific), Agilent TapeStation 2200 using RNA Tape and qRT-PCR (KAPA SYBR® Fast One-Step qRT-PCR Master Mix kit (2x), beta-actin intron flanking transcript, reaction done in triplicates).
|
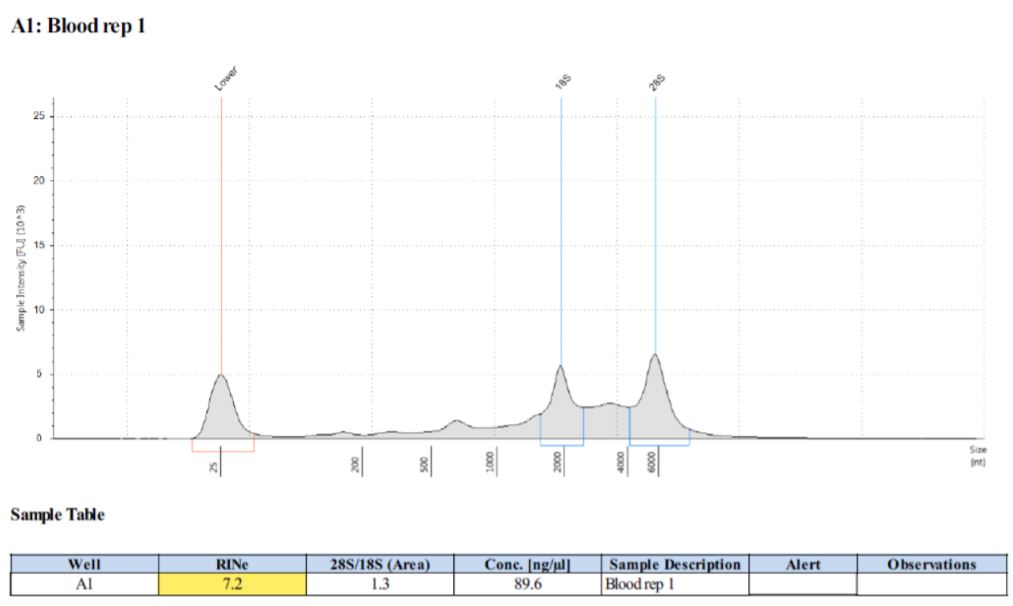
Results High quality RNA is required for many downstream applications. To gain a complete understanding of the RNA extracted by the automated vs. manual protocols, we assessed quality of RNA samples using multiple methods. To estimate nucleic acid purity, NanoDrop calculates the ratio of the absorbance by the nucleic acid to the absorbance of the contaminants. Both manual and automated samples yielded good A260/A280 ratios (1.9–2.14) and acceptable A260/A230 ratios (>1.76). The RNA Integrity Numbers (RIN) calculated by Agilent TapeStation accounts for not only the ratio of 28S and 18S rRNAs but also the electrophoretic trace of RNA, providing an estimate of RNA quality. Both automated and manual methods produced good RIN scores (Figure 5 & 6). To demonstrate the usability of extracted RNA in downstream applications, we carried out qRT-PCR. RNA extracted by both methods amplified in the range of Ct 15-20, indicating the superior quality of RNA (Figure 7). |
Figure 5. Automated RNA samples analyzed on TapeStation 2200. Figure 6. Manual RNA samples analyzed on TapeStation 2200. |
Figure 7. qRT-PCR amplification plots (cycle number vs. florescence) corresponding to automated (a) and manual (b) RNA templates. RNA template concentration 150 ng/uL; X: positive control 200 ng/uL; y: no RT control; z: no template control
Summary
We’ve demonstrated automation of Agencourt RNAdvance Blood Kit on the Biomek i7 Hybrid Genomics Workstation. In terms of quality and quantity, RNA extracted by the automated protocol was comparable to manually extracted RNA. Automation enables fast and efficient extraction of high quality RNA.