Biomek i-Series Automated New England Biolabs NEBNext® Ultra II DNA Library Prep Kit
Biomek Automated Genomic Sample Prep Accelerates ResearchIntroduction
Obtaining high library yields with low input DNA concentration remains a substantial challenge in NGS library preparation. The New England Biolabs NEBNext® Ultra IITM DNA Library Prep Kit addresses this challenge by the use of reformulated kit components, to generate high quality libraries from input DNA amounts, as low as 500 pg. Therefore, the kit is widely used to construct genomic DNA and ChIP-seq libraries from GC rich regions and FFPE samples for Illumina sequencing (NEB Next for Illumina: NGS sample preparation guide). The streamlined workflows of the kit protocol makes it suitable for automation (Figure 1). In this technical note, we demonstrate automated performance of the Kit on the Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation.
When compared to manual pipetting, the NEBNext® Ultra IITM DNA Library Prep Kit automated on a Biomek platform provides:
- Reduced hands-on-time and increased throughput
- Reduction in pipetting errors
- Standardized workflow for improved results
- Quick implementation with ready-to-implement methods
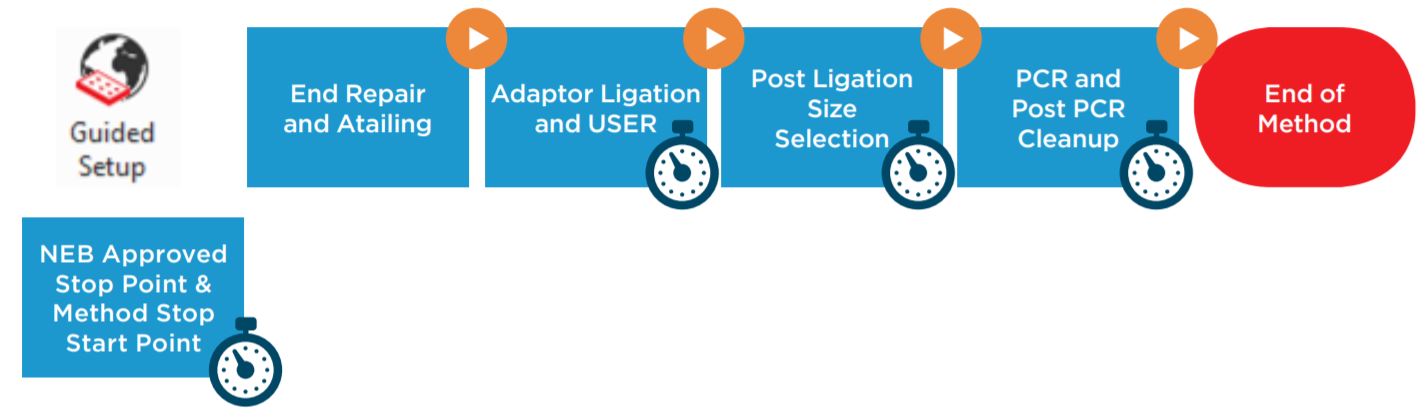
Figure 1. NEBNext® Ultra IITM DNA Library Prep Kit protocol
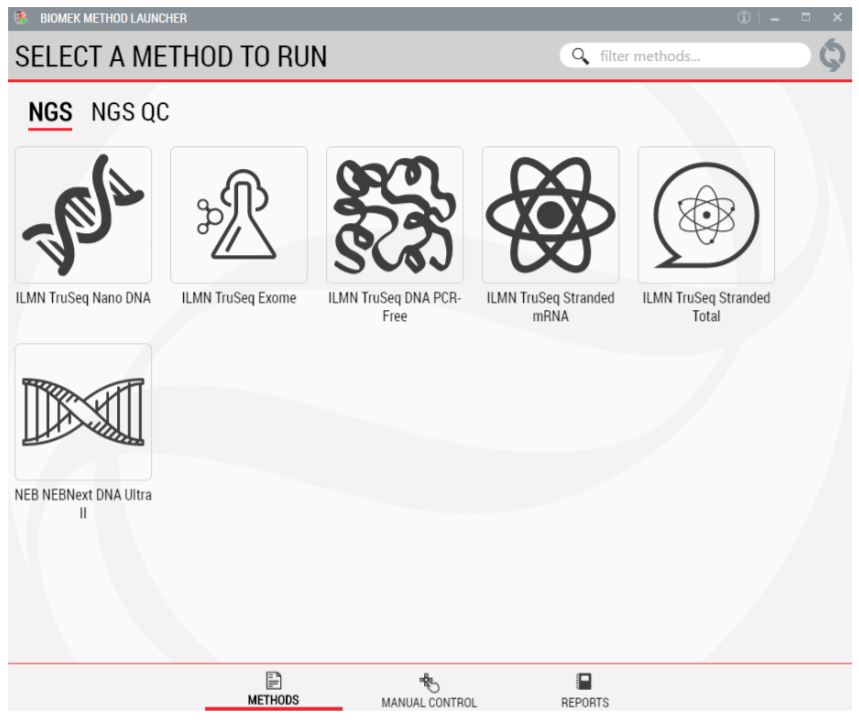
1. Biomek Method Launcher (BML)
BML is a secure and user friendly interface for method implementation (Figure 2). Within BML, the method steps are organized in a modular manner for workflow optimization (Figures 1 and 3). It includes logical start and stop points assigned based on NEB recommendations (Figures 1 and 3). This allows users to recover from errors that occurred during single modules by redoing single modules, without having to start from the beginning of the method. In addition, the users can monitor the progress of the run remotely through BML.
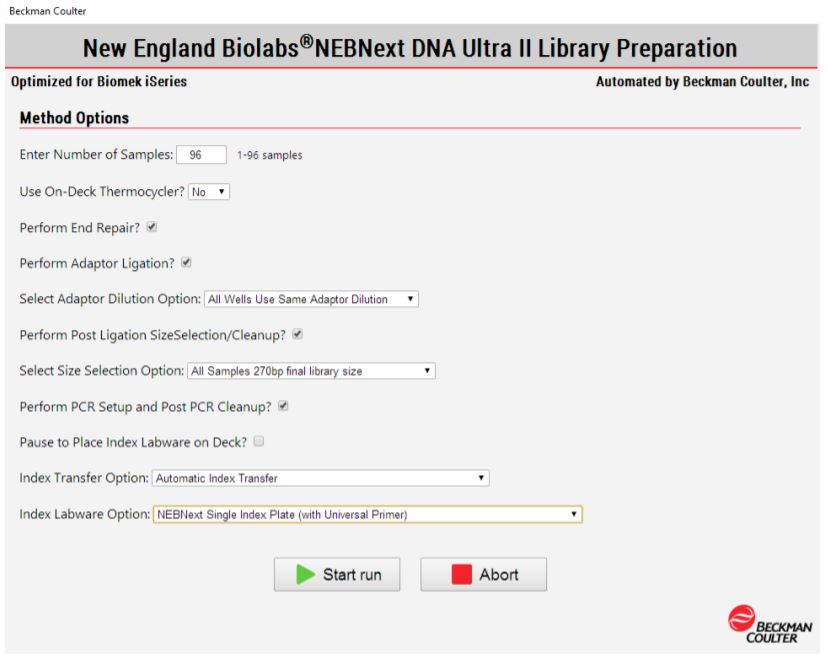
2. Method Options Selector (MOS)
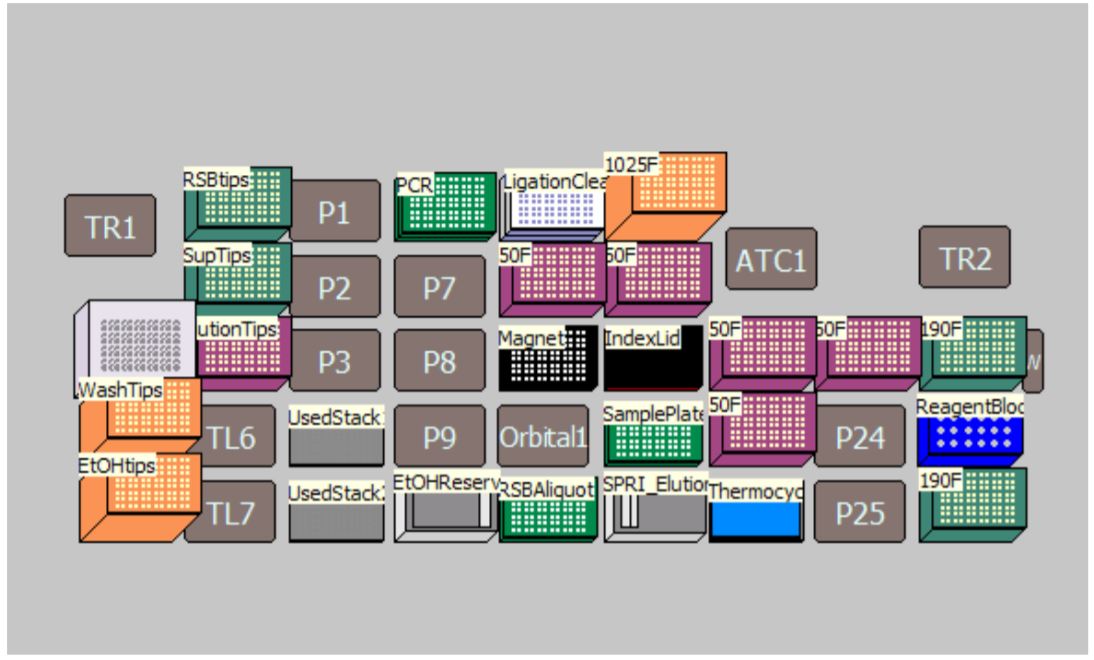
MOS enables selection of sample number and sample processing options (e.g. Index transfer options, size selection options, Adaptor dilution options) to maximize flexibility for the users (Figure 3). The thermocycling steps can be done either off-deck or on-deck using an automated thermocycler (ATC Thermo Fisher; Figure 4). The MOS offers the flexibility to perform per-well size selection and per-well adaptor dilution based on various size selection options provided by NEB.
Figure 2. Biomek Method Launcher provides an easy interface to start the method
Figure 3. Biomek Method Options Selector indicate sample number and processing options
Figure 4. Deck Layout for New England Biolabs NEBNext® DNA Ultra II DNA Library Prep Kit protocol on Biomek i7 Dual Hybrid for 96 samples with on-deck thermocycling option
3. Guided Labware Setup (GLS)
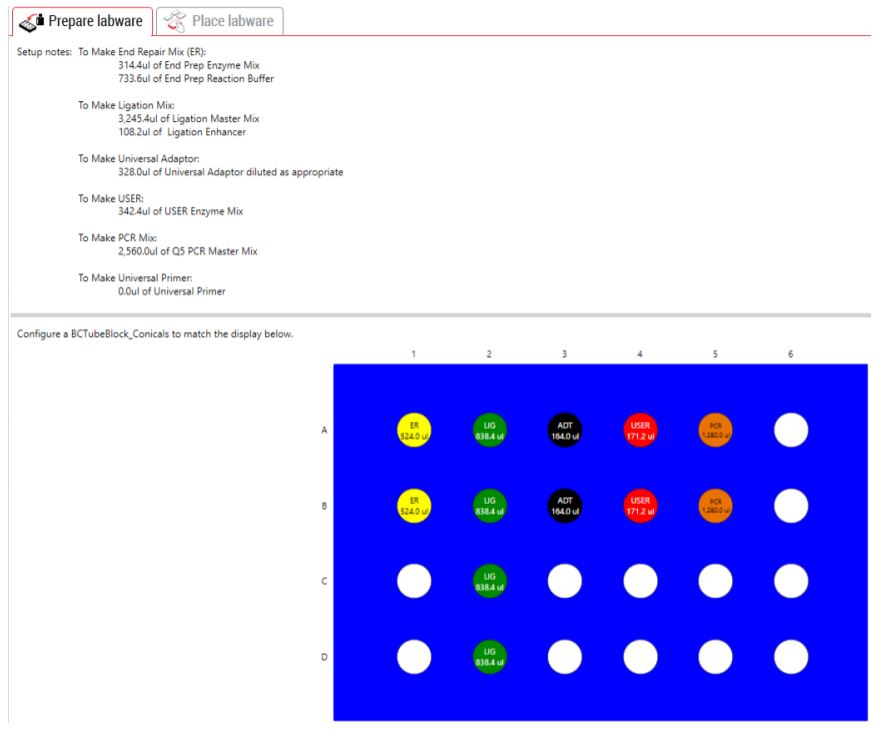
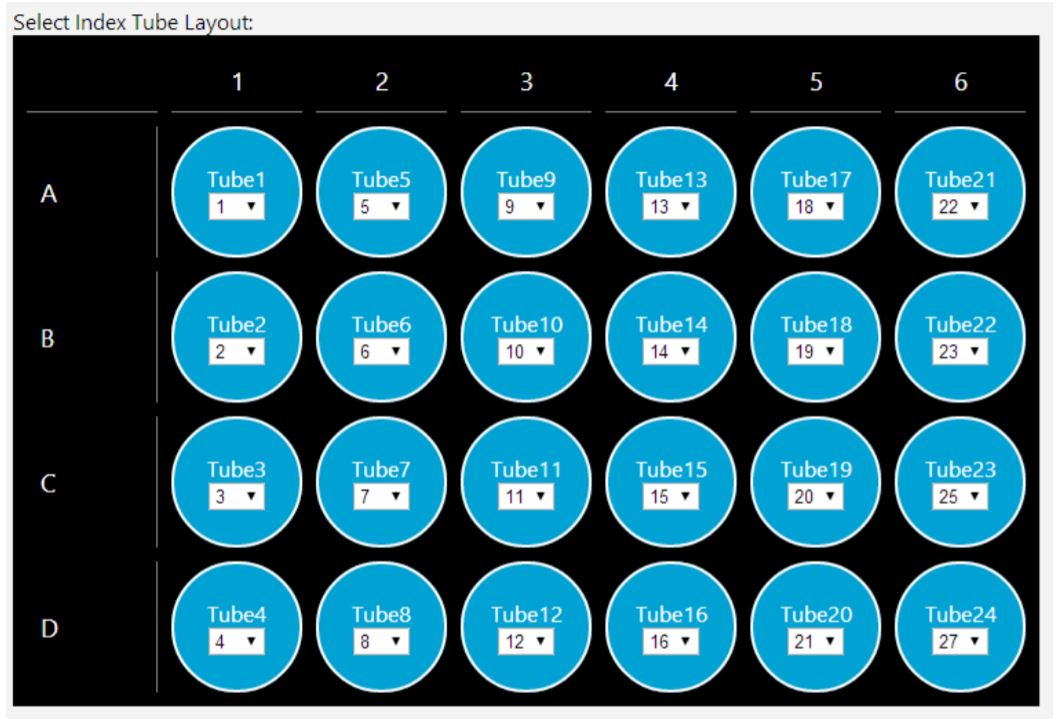
GLS provides the user specific text and graphical setup instructions with reagent volume calculation and step by step instructions to prepare reagents (Figure 5). There is an optional pause step for placing primers on deck after method starts. The automated method supports placing NEB's single index primer tubes, single index primer plate, dual index primer tubes, or a custom primer plate on deck (Figure 6). The custom primer plate option allows the customers to indicate custom primer assignments by providing a .csv file. The dataset driven primer ID capability logs that indicate which primer has been assigned to which sample provide DART(Data Acquisition and Reporting Tool) and LIMS (Laboratory Information Management Systems) friendly sample tracking.
Figure 5. Guided Labware Setup indicates calculated reagent volumes required to make reagent mixes and guides the user for correct deck setup
Figure 6. Guided Labware Setup enables selecting index tube layout
Experimental design
Promega Human gDNA (200 ng/µl) was used for the automation of the NEBNext DNA Library Prep kit protocol on the Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation. The library preparation was performed using two pools of sheared DNA targeting 200 bp and 500 bp (Covaris S220 Focused-Ultrasonicator; 6-7 replicates from each) and amplified using 5 PCR cycles. After the preparation, the libraries were analyzed on Agilent TapeStation 2200 with High Sensitivity D5000 Kit and qPCR with Kapa Illumina Library Quantification Kit.
Results
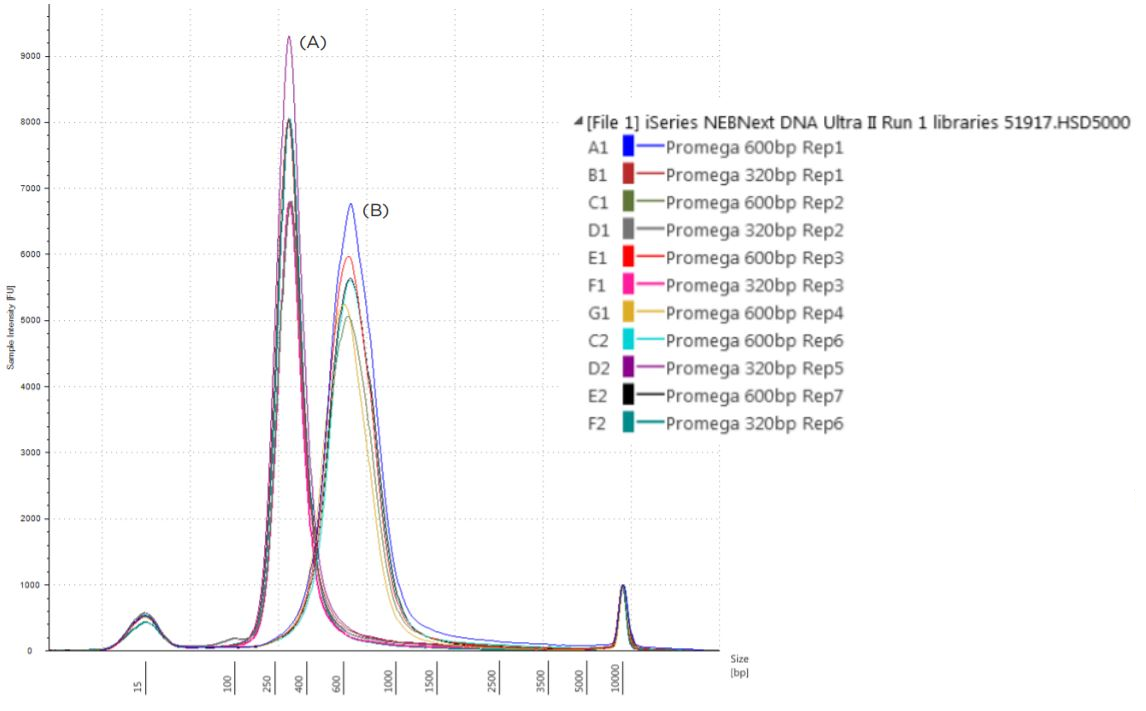
Agilent TapeStation results indicated the prepared libraries are of expected size, after adaptor ligation (Approximately 320 bp total library size for 200 bp fragments and 600-800 bp for 500 bp inserts; NEB Next for Illumina: NGS sample preparation guide; Table 2; Figure 7). Libraries were also assayed using the Kapa Illumina Library Quantification Kit, with qPCR setup being performed using the Kapa Illumina Library Quantification method implemented on the Biomek i-Series (Figure 7).
| Sample ID | Size Selection | TapeStation size (bp) | qPCR Yield (nM) |
| 200 bp Replicate 1 | 320bp | 308 | 35.4 |
| 200 bp Replicate 2 | 320bp | 319 | 24.5 |
| 200 bp Replicate 3 | 320bp | 310 | 22.6 |
| 200 bp Replicate 4 | 320bp | 306 | 32.6 |
| 200 bp Replicate 5 | 320bp | 314 | 25.5 |
| 200 bp Replicate 6 | 320bp | 311 | 36.1 |
| 500 bp Replicate 1 | 600-800bp | 644 | 37.9 |
| 500 bp Replicate 2 | 600-800bp | 622 | 36.4 |
| 500 bp Replicate 3 | 600-800bp | 635 | 22.6 |
| 500 bp Replicate 4 | 600-800bp | 600 | 31.5 |
| 500 bp Replicate 5 | 600-800bp | 645 | 28.5 |
| 500 bp Replicate 6 | 600-800bp | 633 | 33.8 |
| 500 bp Replicate 7 | 600-800bp | 641 | 20.6 |
| 500 bp Replicate 8 | Cleanup Only | 474 | 93.3 |
| No template control | Cleanup Only | 0 | 0 |
Table 2. Library quantification of automated New England Biolabs NEBNext® DNA Ultra II Library Preparation kit protocol using Agilent TapeStation 2200 and Kapa Illumina Library Quantification Kit.
Figure 7. Electropherogram (Sample intensity vs. size in base pairs) of Agilent TapeStation corresponding to 320bp libraries (A) and 600-800 bp (B) libraries.
Summary
We automated the NEBNext® Ultra II TM DNA Library Prep Kit on Biomek i7 Dual Hybrid (Multichannel 96, Span-8) Genomics Workstation. Our quality assessments indicate that the prepared libraries are suitable for sequencing. Our Automated protocol increases the library preparation efficiency by cutting down the hands on time. The protocol can be executed using the Biomek Method Launcher, a user friendly interface that allows the customers to monitor the progression of the method remotely.