g-Max: Added Capabilities to Beckman Coulter's versatile Ultracentrifuge Line
Content Type: Application Note
Authors:
Chad Schwartz, Ph.D., Application Scientist I
Randy Lockner, M.S., Centrifugation Marketing
Randy Pawlovich, Director of Product Management & Strategy
Beckman Coulter, Inc., Indianapolis, IN 46268
The g-Max System adds several valuable capabilities to your Beckman Coulter ultracentrifuge rotors:
- Flexibility of Volumes
- Maximized Efficiency
- Heat-Sealed Technology
Flexibility of Volumes
Based on a unique approach to tube support within the rotor cavities, the g-Max System allows smaller volumes to be run in vertical, fixed-angle, and swinging bucket rotors with increased efficiency and no reduction in centrifugal acceleration. This innovative system uses patented Beckman Coulter Quick-Seal1 bell-top polypropylene tubes and floating spacers. Unlike conventional sleeve-type adapters, the g-Max spacers “float” on top of the tube, keeping the sample at the maximum radius of the tube cavity. This means you can run smaller volumes in your rotors with higher efficiency.
Maximized Efficiency
For pelleting applications, your separations will take less time. By reducing the pathlength that a particle is required to travel before pelleting at the bottom of the tube, g-Max technology can significantly reduce researchers’ time and increase efficiency.
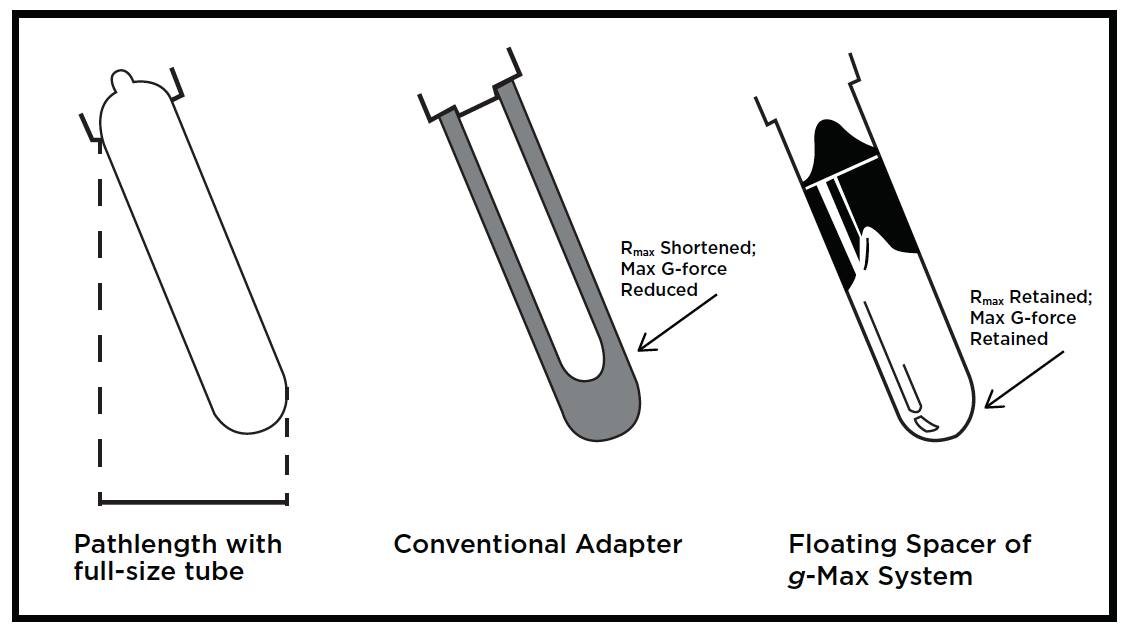 |
| Figure 1: The g-Max System in fixed-angle rotors provides significant advantages over both full-size tubes and conventional adapters. |
Heat-Sealed Technology
Quick-Seal tubes are designed for use in all swinging bucket, vertical tube, near-vertical tube, and most fixed-angle rotors. The tubes are available in many sizes and in 3 different designs—dome-top, bell-top, and konical bell-top. Quick-Seal tubes are especially useful in runs where biohazards pose a potential contamination problem. There is no need for caps—the tubes are heat-sealed, resulting in a very reliable seal, keeping scientists safe and our environment clean.
Fixed-Angle Rotors
BSA stock solution was prepared by dissolving BSA powder into PBS to an absorbance of 1.0 OD at 280nm. The OD of the samples was measured using a Beckman-Coulter DU730 UV/Vis Spectophotometer with a 10mm pathlength. BSA was diluted to working concentrations of 0.4 and 0.9 OD in PBS.
A good measure of the efficiency of a rotor or tube is the k-factor—which is a guide to the time, t (in hours), required to pellet a particle of known sedimentation coefficient, s (in Svedberg units)—where t = k/s. Rotor efficiency increases as the k-factor is lowered, as k-factor is directly proportional to run time. Table 1 shows an example of how the g-Max System provides higher performance than a conventional adapter or full-size tube for the Type 70.1 Ti and Type 90 Ti rotors. By comparing the k-factors, pelleting operations would take one-third to one-fourth the time with g-Max technology versus the sleeve-type competitor. Furthermore, many sleeve-type conventional adapters cannot be used to maximum rotor speed due to stability issues. The g-Max System also offers reductions in run times over full-size tubes. For example, the k-factor of the Type 90 Ti with a 13.5-mL tube is 25, compared to 14 for a 6.3-mL tube.
| Table 1. Performance Comparisons by Rotor Type. | ||||
| Adapter Type | Volume (mL) | Max. Speed (rpm) | k-factor | Max. RCF |
| Type 70.1 Ti | ||||
| Full-Size | 13.5 | 70,000 | 36 | 450,000 |
| Conventional | 6.5 | 50,000 | 60 | 212,000 |
| g-Max | 6.3 | 70,000 | 24 | 450,000 |
| Type 90 Ti | ||||
| Full-Size | 13.5 | 90,000 | 25 | 694,000 |
| Conventional | 6.5 | 50,000 | 69 | 197,000 |
| g-Max | 6.3 | 90,000 | 14 | 694,000 |
Vertical Tube Rotors
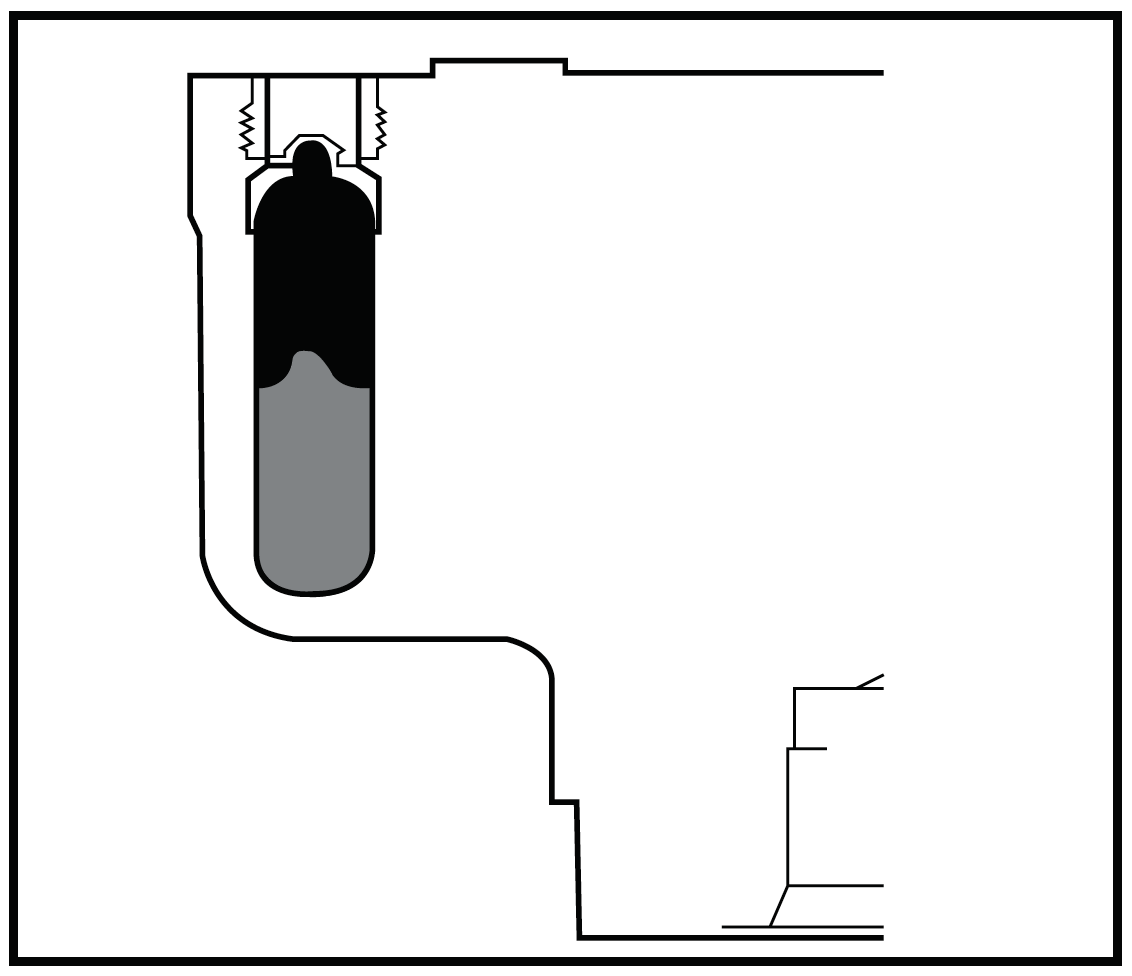 |
| Figure 2: Vertical tube rotors are also compatible with g-Max tubes, providing flexibility of volumes to your experimental design. |
Vertical tube rotors are widely used for gradient separations due to their short run times. Incorporating the g-Max System, all Beckman Coulter vertical tube rotors can run 2 or more tube sizes. A common separation performed in these rotors is plasmid DNA preparations. Although the g-Max System will have no effect on run times because there is no change in pathlength, g-Max technology allows for flexibility of volume in vertical rotors which can help researchers save critical samples or mitigate a large dilution effect (Figure 2).
Swinging Bucket Rotors
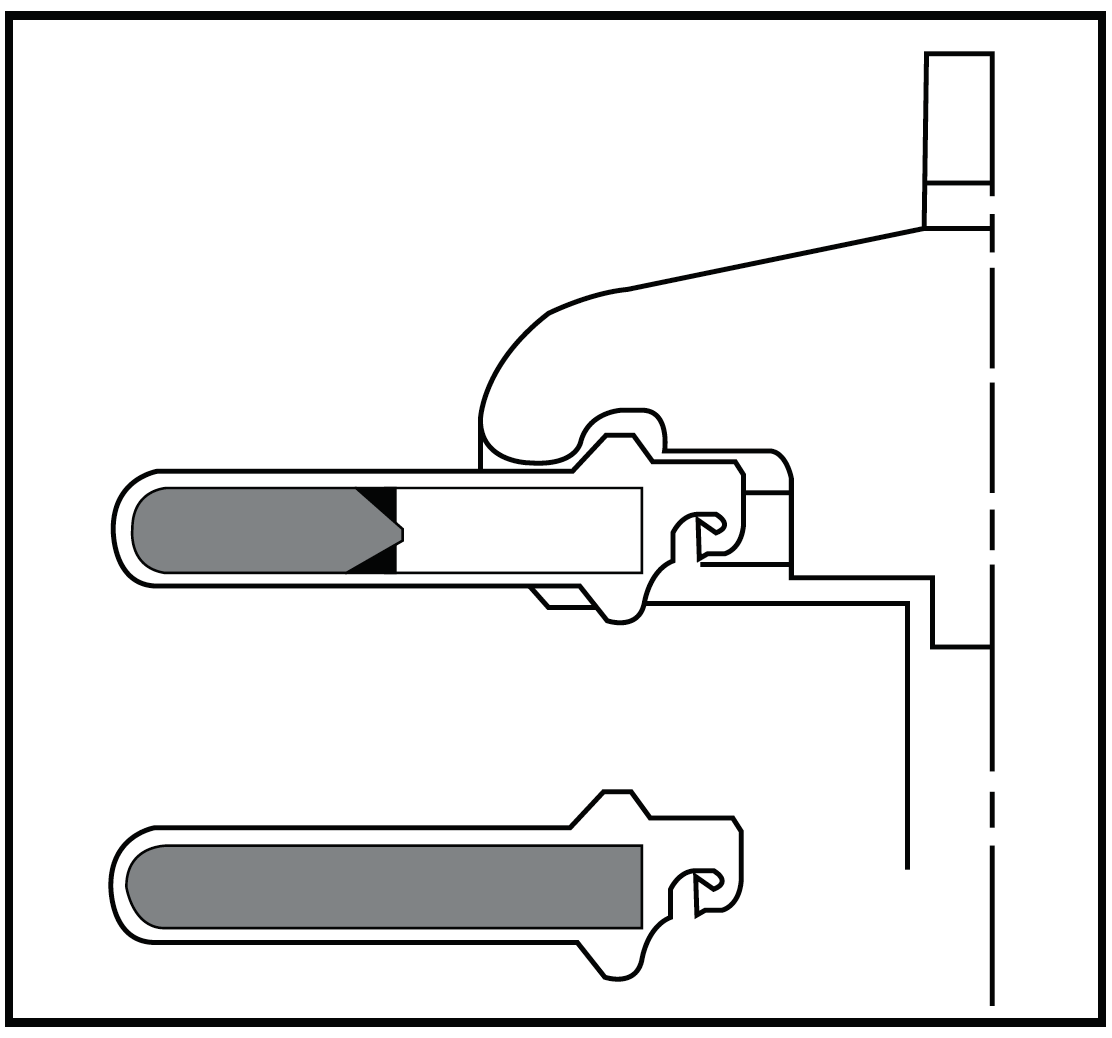 |
| Figure 3: Swinging bucket rotors offer the most significant advantages with the g-Max System.. |
One of the major advantages of the g-Max System, especially in swinging bucket rotors, is for secondary containment. Because Quick-Seal tubes have no caps and their seal is so reliable, they are uniquely useful for biohazardous or radioactive applications. Utilizing shorter pathlengths, as permitted by the g-Max floating spacers, results in some of the best time-savings for separations where large interband volumes are not critical. The smaller pathlength tubes provide flexibility of volumes, shorten run times, and also decrease the interband volumes (Figure 3).
Benefits Overview of Rotor Type
The g-Max System offers advantages over common tubes and sleeve adapters in all rotor types, but the largest benefits are seen in both the fixed-angle and swinging bucket rotors. Table 2 highlights these advantages among the three main types of rotors.
| Table 2. Benefits of g-Max System by Rotor Type. | |||
| Rotor Type | Flexibility of Volume | Shorter Run Time | Heat-Sealed Technology |
| Vertical Tube | X | X | |
| Fixed-Angle | X | X | X |
| Swinging Bucket | X | X | X |
Example of Time Savings: Exosome Purification
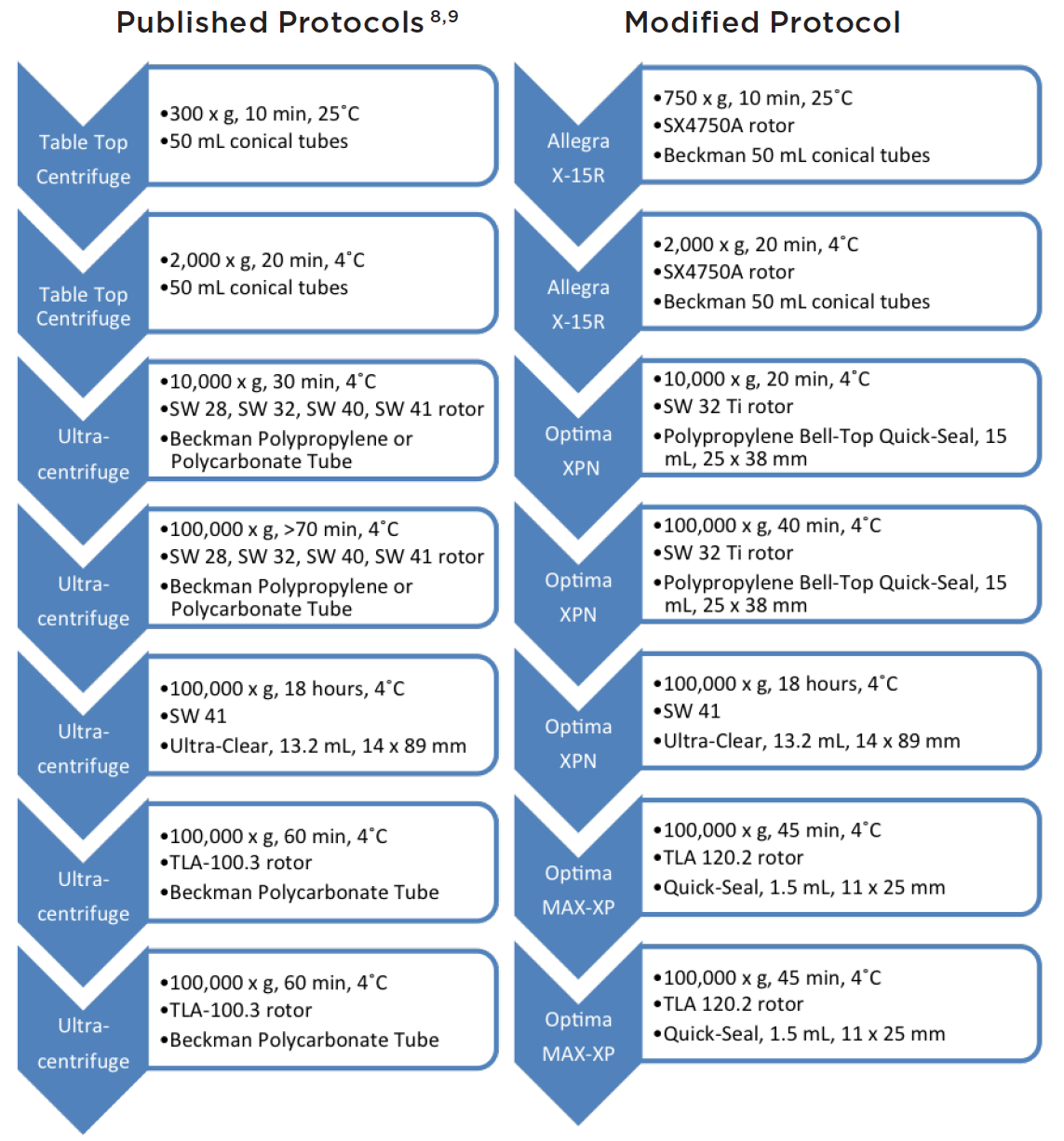 |
| Figure 4: Comparative protocols showing critical time savings using g-Max technology. Over 1 hour of time was saved in the centrifuge using the modified protocol. |
Research involving exosomes is rapidly expanding with a vast increase in the number of publications recently. Exosomes are small vesicles between 30 and 100 nm, demonstrated to be released by a wide variety of cell types, exhibiting a multitude of functions, and often involved in cancer.2-6
An improved and more efficient isolation protocol for exosomes is critical to advancing this exciting field.7 Here, we present a method using g-Max technology that improves processing time, and generates a high-yield, highly pure collection of exosome vesicles.
Typically, cultured cells are centrifuged and pelleted in 2 to 3 steps at low speed to remove debris and dead cells. Next, using an ultracentrifuge at speeds of 100,000 x g, exosomes and contaminating proteins are pelleted over an extended period of 90 minutes.8 The pellet is subsequently resuspended in buffer (usually PBS) and layered on top of a density gradient and spun for 18 hours.9 Fractions are collected, pelleted separately by 2 additional spins of 100,000 x g for 1 hour each, and characterized by either electron microscopy, size particle analyzers, or Western blots to determine purity and size. The current method is quite tedious and time-consuming, but generates highly pure exosomes of proper size and shape.
Here, we present—using advanced g-Max technology to shorten pathlengths but retain high g-forces—a method to cut lab time significantly (Figure 4).
Revised Method
In our revised protocol, cells were cultured from frozen stock and their viability was measured by Beckman Coulter’s Vi-CELL. The Vi-CELL provides accurate sizing, count, and viability of cells in culture using the well-established Coulter Principle. After obtaining highly viable cells, 25 mL of cell culture samples (6 x 105 cells/mL) were added to 50 mL conical tubes, and placed in a SX4750A rotor with 50 mL conical adapters in the Allegra X-15R tabletop centrifuge, and spun for 10 minutes at 750 x g. The supernatant was subsequently recovered and spun at 2,000 x g for 20 minutes. This supernatant was then spun again at 10,000 x g for 20 minutes in the Optima XPN ultracentrifuge, equipped with a SW 32 Ti rotor using Quick-Seal 15 mL tubes to remove cell debris. Again, the supernatant was recovered, filtered through a 0.22 μm membrane, and spun at 100,000 x g for 40 minutes in 15 mL Quick-Seal tubes equipped with g-Max adapters. This time, the supernatant was aspirated and the pellet was recovered by resuspension in 1x PBS.
Beckman Coulter’s Biomek 4000 Laboratory Automation Workstation was used to provide a quick, consistent, and reproducible method for layering a density gradient with the volumes and density shown in Figure 5. The resuspended exosomes (1 mL) were then layered on top of the density gradient, and spun at 100,000 x g at 4˚C for 18 hours.
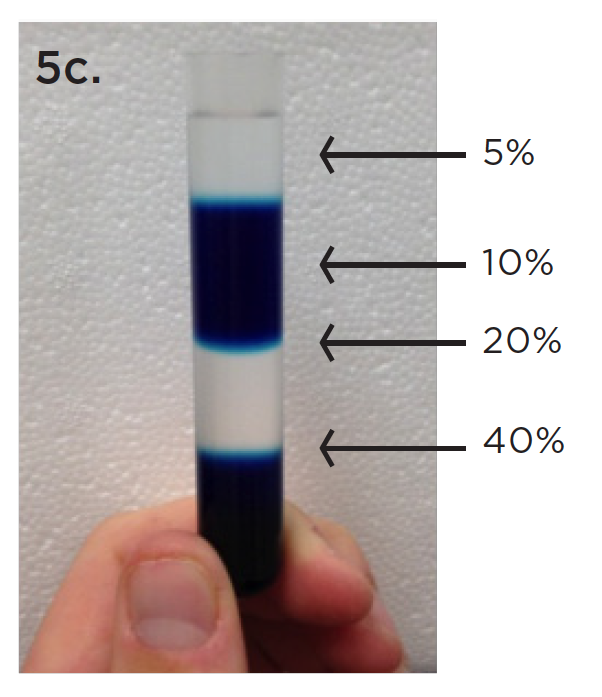
500 μl fractions were collected from the top and then combined with every third fraction for a total volume of 1.5 mL per combined fraction. Samples were then added to Quick-Seal 1.5 mL tubes with g-Max adapters and spun at 100,000 x g in the Optima MAX-XP ultracentrifuge for 45 minutes at 4˚C. Finally, the pellet was resuspended in PBS and spun at 100,000 x g in the Optima MAX-XP ultracentrifuge for an additional 45 minutes, in the same 1.5 mL Quick-Seal tubes with g-Max adapters, to get rid of excess Opti-Prep. The final pellet was resuspended in 100 μl PBS and frozen at -20˚C prior to analysis for purity.
As shown in Figure 6, Beckman Coulter’s DelsaMax System was used to characterize the size of particles between our two methods. All eight 1.5 mL fractions were analyzed by DLS with rigid parameters for both the general, published protocols for separation and the revised g-Max procedure. Early fractions 1–12 had high protein contamination as indicated by peaks below 10 nm in diameter (data not shown). Again, later fractions 16–24 had strong protein contamination and also contained particles over 250 nm in diameter, suggesting contamination of cell debris or other large molecules (data not shown).
However, fractions 13–15 for both the published protocol and the revised g-Max protocol contained particles of size indicative of exosomes (around 30 to 100 nm diameter). This suggests that the density gradient properly separated the exosomes from contaminants and that both protocols are similarly efficient in isolating exosomes. This data supports our method and the use of g-Max technology to save critical time, yet still generate accurate and reproducible results.
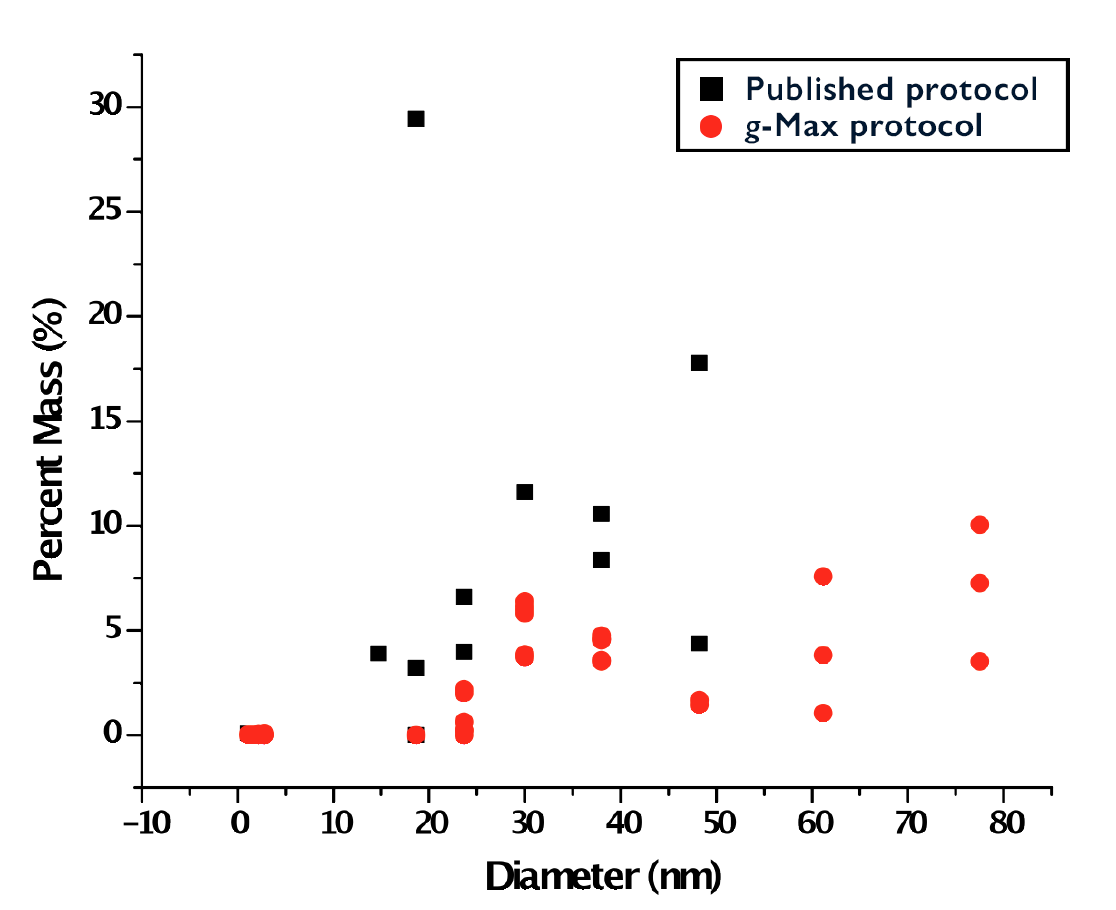 |
| Figure 6: DelsaMax characterization of resuspended pellets, following density gradient separation, where the particle size using g-Max technology (red) (n=9) is compared to the published protocol (black) (n=3). Both protocols generate particles of size less than 20 nm suggesting some protein contamination. However, particles between 30 nm and 100 nm represent the appropriate size of exosomes. |
Rotor Compatibility
g-Max tubes can be utilized in a variety of rotors as outlined in the table below. Many tubes are compatible across the 3 main rotor types.
| g-Max Compatibility | |||
| Nominal Filling Capacity (mL) | Nominal Size (mm) | Part No. | Rotors* |
| Quick-Seal Polypropylene Tubes | |||
| 1.5 | 11x25 | 344624 | SW 60 Ti, TLA-120.2, MLA-130, MLA-150, TLS-55 |
| 2.0 | 11x32 | 344625 | SW 60 Ti, TLA-120.2, MLA-130, MLA-150, TLS-55 |
| 2.0 | 13x25 | 345829 | Types 100 Ti, 50.4 Ti, 50.3, NVT 100, NVT 90, NVT 65.2, VTi 90, VTi 65.2, SW 50, TLA-110, TLA-100.3 MLS-50 |
| 3.5 | 13x30 | 349621 | Types VTi 90, 100 Ti, TLA-110, TLA-100.3 |
| 3.5 | 14x25 | 355870 | SW 41 Ti, SW 40 Ti |
| 4.2 | 16x32 | 356562 | Types 90 Ti, 70.1 Ti, 65, 40, SW 32.1 Ti, SW 28.1, MLA-80, MLN-80, MLA-55 |
| 5.1 | 13x51 | 362248 | Types VTi 90, 100 Ti |
| 5.9 | 14x47 | 355537 | SW 41 Ti, SW 40 Ti |
| 6.3 | 16x44 | 345830 | Types 90 Ti, 80 Ti, 70.1 Ti, 65, 50 Ti, 50, 40, NVT 65, VTi 65.1, SW 32.1 Ti, SW 28.1, MLA-80, MLN-80, MLA-55 |
| 8.0 | 16x57 | 344621 | Types 50, NVT 65, VTi 65.1 SW 32.1 Ti, SW 28.1, MLA-80, MLN-80 |
| 10.0 | 16x67 | 344622 | Types 90 Ti, 80 Ti, 70.1 Ti, 65, 50 Ti, 40, NVT 65, VTi 65.1, SW 32.1 Ti, SW 28.1, MLA-55 |
| 15.0 | 25x38 | 343664 | Types 70 Ti, 60 Ti, 55.2 Ti, 50.2 Ti, VTi 50, SW 32 Ti, SW 28, MLA-50 |
| 27.0 | 25x64 | 343665 | Types 70 Ti, 60 Ti, 55.2 Ti, 50.2 Ti, 42.1, VTi 50, SW 32 Ti, SW 28, MLA-50 |
| 33.5 | 25x83 | 344623 | Types 70 Ti, 60 Ti, 55.2 Ti, 50.2 Ti, SW 32 Ti, SW 28 |
| Quick-Seal Polypropylene konical Tubes | |||
| 1.3 | 11x35 | 358655 | SW 60 Ti |
| 4.0 | 14x48 | 358650 | SW 41 Ti |
| 8.4 | 25x38 | 358652 | SW 32 Ti, SW 28 |
| 22.5 | 25x76 | 358654 | SW 32 Ti, SW 28 |
| 28.0 | 25x83 | 358651 | SW 32 Ti, SW 28 |
| Quick-Seal Ultra-Clear Tubes | |||
| 15.0 | 25x38 | 344324 | Types 70 Ti, 60 Ti, 55.2 Ti, 50.2 Ti, VTi 50, MLA-50 |
| 27.0 | 25x64 | 344323 | Types 70 Ti, 60 Ti, 55.2 Ti, 50.2 Ti, VTi 50, MLA-50 |
| Thin-Wall Polypropylene, konical Tubes | |||
| 1.5 | 11x35 | 358117 | SW 60 Ti |
References
- U.S. Patent Nos. 4,301,963; 4,304,356; 4,290,550; British Patent No. 2,021,982; Canadian Patent No. 1,132,509; Japanese Patent No. 1,469,153; Italian Patent No. 1,121,772.
- Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol Lett. 107(2); 102–8: (2006). doi:10.1016/j.imlet.2006.09.005
- Duijvesz D et al. Exosomes as biomarker treasure chests for prostate cancer. European urology. 59.5; 823–831: (2011).
- Jero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15(1); 80–8: (2007). doi:10.1038/sj.cdd.4402237
- Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011; 1–11: (2011). doi:10.1155/2011/842849
- Azorsa DO. The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol. 2; 38: (2012). doi:10.3389/fonc.2012.00038/abstract
- Simpson RJ, Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J Proteomics Bioinform. 5: (2012).
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 3.22: (2006). doi:10.1002/0471143030
- Tauro BJ et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 56.2; 293–304: (2012).
CENT-447APP08.14.A