A complete workflow for high-throughput isolation of DNA and RNA from FFPE samples using Formapure XL Total on the KingFisher™ Sample Purification System: an application for robust and scalable cancer research and biomarker discovery
Brittany Niccum, PhD, Beckman Coulter Life Sciences, Indianapolis, INSummary
Formalin-fixed paraffin-embedded tissues are an invaluable resource for histological and genetic testing for cancer. Current next-generation sequencing (NGS) technologies and extraction methods make it possible to study and identify cancer-causing alterations at the genomic and transcriptomic levels. However, challenges exist in most FFPE workflows, especially when high-throughput extractions are a requirement. First, FFPE samples generally provide low-quality nucleic acids, especially the RNA, as RNA undergoes more severe degradation and chemical and covalent modifications due to the effects of formalin-fixation than DNA. Second, consistent results for most high-throughput and automated extraction methods for FFPE are difficult to achieve due to inherent differences in the variety of tissue and disease types. A solution that offers the ability to apply high-throughput and reliable genomic applications to FFPE samples can help accelerate cancer research and biomarker discovery.
This application note demonstrates the use of FormaPure XL Total, a total nucleic acid extraction kit for FFPE samples, in conjunction with the KingFisher™ Duo Prime Sample Purification System as a potential solution that mitigates some of the challenges with FFPE workflows. FormaPure XL Total has been demonstrated to obtain high quality FFPE-derived nucleic acids . By automating FormaPure XL Total chemistry on the KingFisher™ Duo Prime the difficulty of working with FFPE tissue is eased. Automating the chemistry can also reduce the risk of human error, reduce hands-on time and total time; therefore giving the user the ability to run more samples in a day.
Method
Manual processing
In this study three FFPE tissue samples were used to assess the differences between manual and semi-automated RNA and DNA extraction. 10 µM curls were cut and three samples consisting of 3 curls were processed both manually and on automation. For each tissue type 450 µL of mineral oil was added to each sample. The samples were then incubated at 80°C for 5 minutes. Then 200 µL of LBD was added and the samples were centrifuged according to the protocol. The samples were incubated again at 80°C for 5 minutes. Following the incubation the tubes were allowed to cool for 5 minutes before the addition of 30 µL of proteinase K. The samples were then incubated at 60°C for 2 hours. After the 2 hour incubation 100 µL of the lysate was removed and added to a KingFisher™ Duo Prime 96 well plate for RNA processing.
The remaining lysate was incubated at 60°C for another hour followed by an 80°C incubation for 1 hour. After the incubation 100 µL was removed and added to a KingFisher™ Duo Prime 96 well plate. RNase A was added to the 100 µL lysate and mixed 10 times. The mixture was incubated at room temperature for 5 minutes. The plate was then moved to the KingFisher™ Duo Prime for DNA processing.
Automated processing
The parameters for mix times and speeds and the collect times were optimized for the magnetic beads on the KingFisher™ Duo Prime. The optimized parameters and reagent volumes for RNA processing are in Table 1 and for DNA processing are in Table 2. The automation for RNA extraction is a two-step process. First the protocol is automated up to the DNase I treatment. After the incubation for the DNase treatment, the user must add 150 µL of RBA; the automated processing can proceed on from there.
| RNA
Purification
Step |
Plate Row |
Reagent |
Volume (μL) |
Automation parameters |
||
| Mixing time/Mixing Speed/ Pause Time | Collect Count/ time [s] | |||||
| DNase Treat | A | DNase I solution | 100 μL | 20 sec / medium / 20 min | 5 / 30 sec | Heating 37°C |
| Bind |
B |
BBA | 150 μL | 20 sec / medium / 5 min |
5 / 30 sec |
|
| Lysate | 100 μL | |||||
| Wash | C | 80% Ethanol | 375 μL | 20 sec / medium / 5 min | 5 / 30 sec | |
| Wash | D | 80% Ethanol | 375 μL | 20 sec / medium / 5 min | 5 / 30 sec | |
| NA | E | NA | NA |
NA |
||
| NA | F | NA | ||||
| NA | G | KingFisher™ Duo 12-Tip Comb | ||||
| Elute | H | Nuclease Free Water | 40 μL | 20 sec / medium / 5 min | 5 / 30 sec | |
Table 1. RNA processing
| DNA
Purification
Step |
Plate Row |
Reagent |
Volume (μL) |
Automation parameters |
|
| Mixing time/Mixing Speed/ Pause Time | Collect Count/ time [s] | ||||
| Elute | A | Nuclease Free Water | 40 μL | 20 sec / medium / 20 min | 5 / 30 sec |
| Bind |
B |
BBA | 150 μL | 5 min / medium / NA |
5 / 30 sec |
| Lysate | 100 μL | ||||
| Rnase A | 2.5 μL | ||||
| Wash | C | WBA | 200 µL | 20 sec / medium / NA | 5 / 30 sec |
| Wash | D | 80% Ethanol | 375 µL | 20 sec / medium / NA | 5 / 30 sec |
| NA | E | KingFisher™ Duo 12-Tip Comb | NA |
NA |
|
| NA | F | Empty | |||
| NA | G | Empty | |||
| NA | H | Empty | |||
Table 2. DNA Processing
Results
DNA and RNA was extracted from 3 10 µM curls of breast, intestine, and lung FFPE tissue. The average yields are shown in figure 1. The yields for RNA and DNA are similar between manual and automation. The differences in yields with RNA from lung tissue and DNA from breast tissue can most likely be attributed to differences in tissue distribution within an FFPE block.
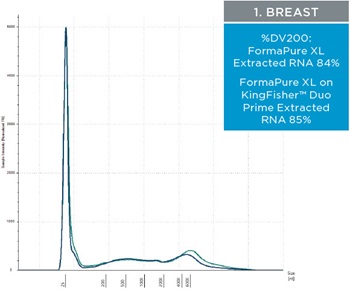
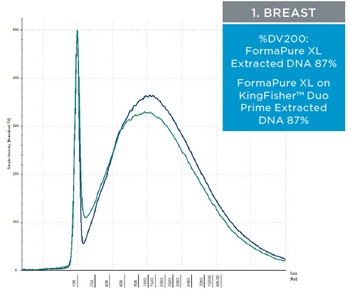
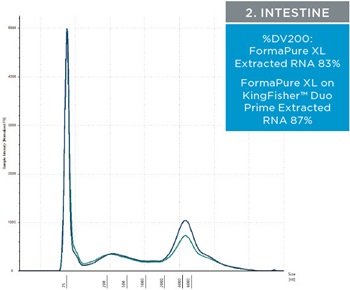
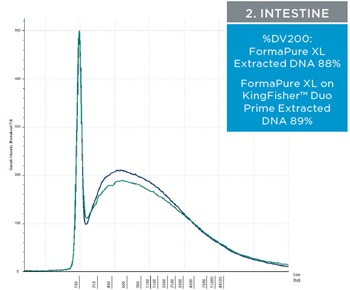
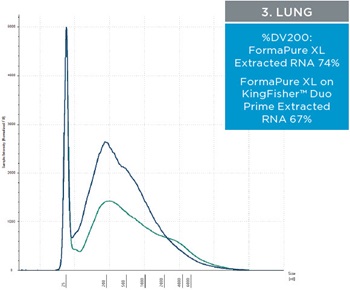
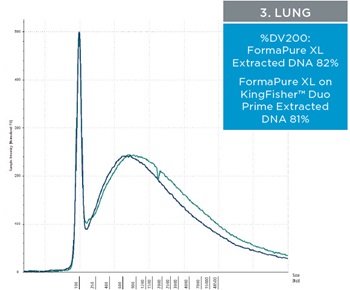
The percent DV200 value is an important quality score to keep in mind for NGS. The percent DV200 is the percent of nucleic acid fragments that are larger than 200 nucleotides. In making FFPE tissue the formalin can cross link DNA or RNA to proteins. This causes the nucleic acids to fragment and to be very delicate after and during extraction. Various external forces can cause DNA and RNA to fragment more, such as the pressure it takes to move through a column matrix or vigorous pipetting. The percent DV200 values for RNA and DNA extracted from the three tissues using FormaPure XL Total manually and on the KingFisher™ Duo Prime are not significantly different from each other (t test RNA p=0.8 and DNA p=0.3). The average percent DV200 values for RNA for each tissue type is reported in table 3 and representative traces are in figure 2; all percent DV200 values are greater than 65%. The average percent DV200 values for DNA are presented in table 3 and representative traces are in figure 3.
Lastly, we compared the hands-on time between the manually and semi-automated methods of FormaPure XL Total. For manually processing 12 samples, the hands-on time is about 3.5 hours while for processing on the KingFisher™ Duo Prime is about 1.5 hours (table 4). Total time is also decreased by about 1.5 hours with processing on the KingFsher™ Duo Prime.
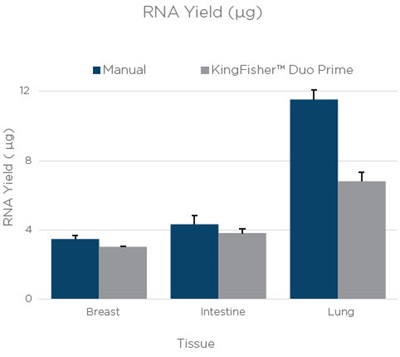
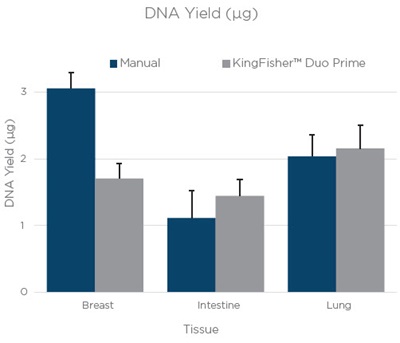
Figure 1. The average yield from three technical replicates of RNA and DNA extraction done manually or on a KingFisher™ Duo Prime. The bars are an average yield of three technical replicates as calculated by Quant-iT assay (Thermo Fisher Scientific). Error bars are the standard deviation of three technical replicates.
| RNA |
DNA |
|||
| Manual | KingFisher™ Duo Prime | Manual | KingFisher™ Duo Prime | |
| Breast | 84.7 | 88.5 | 88.8 | 86.3 |
| Intestine | 76.3 | 84.8 | 86.8 | 88.7 |
| Lung | 72.4 | 69.5 | 83.0 | 82.2 |
Table 3. The average percent DV200 values for RNA and DNA extracted from three tissue types. RNA and DNA was extracted from three 10 µM curls manually and using the KingFisher™ Duo Prime with FormaPure XL Total. The percent DV200 values were not significantly different between the KingFisher™ Duo Prime and manually
 |
 |
 |
 |
 |
 |
 |
|
| Figure 2. Representative electropherograms of RNA isolated using FormaPure XL Total manually (green traces) and using the KingFisher™ Duo Prime (blue traces) from breast, intestine and lung FFPE samples are shown | Figure 3. Representative electropherograms of DNA isolated using FormaPure XL Total manually (green traces) and using the KingFisher™ Duo Prime (blue traces) from breast, intestine and lung FFPE samples are shown. |
Conclusion
This study demonstrates a solution that enables a semi-automated workflow for reliable isolation of DNA and RNA from single FFPE samples. The data presented suggests that the yield and integrity of DNA and RNA extracted from FFPE samples are comparable between manual and semi-automated extractions using FormaPure XL Total. There are several advantages of using KingFisher™ Duo Prime to semi-automate the FormaPure XL Total chemistry. First, the semi-automated workflow does not significantly alter the yield and quality of DNA and RNA compared to a manual workflow as shown with the three tissue types. Up-front manual processing of sample deparaffinization, tissue digestion, decrosslinking and lysate splitting enables researchers to identify and resolve many of the challenges that typically lead to sample loss or drop-outs.
Second, once these upstream steps are meticulously performed, the latter half of the extractions, which involves the bind, wash and elute steps, can be automated in a fraction of the time that it takes to process manually. Semi-automated processing of FFPE samples using FormaPure XL Total and KingFisher™ Duo Prime provides a viable solution to accelerate biomarker discovery. Table 4 outlines the three main differences between the manual vs semi-automated workflows.
| Throughput per run | Hands-on Time (12 samples) | Processing time (12 samples) | |
| Manual | 24 | ~3.5 hours | 6.5 hours |
| Semi-automated KingFisher™ Duo Prime | 12 | ~1.5 hours | 5 hours |
Table 4. The time and throughput abilities for both manual and semi-automated extraction.
Beckman Coulter makes no warranties of any kind whatsoever express or implied, with respect to this protocol, including but not limited to warranties of fitness for a particular purpose or merchantability or that the protocol is non-infringing. All warranties are expressly disclaimed. Your use of the method is solely at your own risk, without recourse to Beckman Coulter. Not intended or validated for use in the diagnosis of disease or other conditions. This protocol is for demonstration only, and is not validated by Beckman Coulter.
KingFisher, KingFisher Duo Prime, and Quant-iT™ dsDNA Assay (PN: Q33120) are trademarks of Thermo Fisher Scientific, Inc.

