Metal colloid purification and concentration using ultracentrifugation
Metal colloids are metal nanoparticles, and are widely used in ink jet wiring technology, catalysts, and life science applications. Metal colloid nanoparticles are primarily synthesized using atomization or chemical reduction, and when using these methods, impurities or agglomerates may be present. In the experiment this time, we will introduce a method to purify and concentrate a synthesized metal colloid using ultracentrifugation.
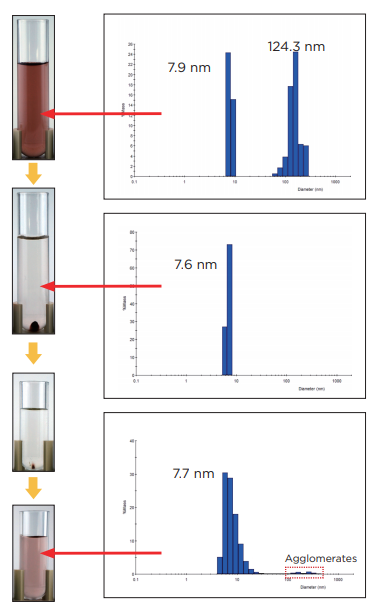 Sample prior to centrifugation
Sample prior to centrifugation
Silver colloid particles prior to centrifugation There is a particle diameter distribution with impurities and agglomerates present.
Centrifuge operation 1: Purification using ultracentrifugation
After centrifugation at 500,000 xg / 30 min, the supernatant was collected and the particle diameter was measured. Based on these results, it is believed that the primary particles are the colloid particles suspended within the solution. The impurities and agglomerates precipitated to the bottom of the tube, forming a deposit.
Centrifuge operation 2: Concentration using ultracentrifugation
The supernatant from centrifuge operation 1 was collected and further centrifuged at 1,000,000 xg / 60 min. The supernatant was clear and colorless, indicating that the silver colloid particles were no longer present. The deposit that was formed consisted of the silver colloid primary particles. Approximately 1/3 of the bottom layer including the deposit described above was collected and re-dispersed. This particle diameter distribution was approximately the same as prior to concentration, confirming that the silver colloid particles had been concentrated.
Discussion
It is clear that impurities and agglomerates were present in the silver colloid particles prior to the centrifuge processing. After centrifuge operation 1 (500,000 xg / 30 min), it was possible to confirm that the impurities and agglomerates were removed and that the material had been purified based on the results of the DelsaMax PRO measurements. Further, by further centrifuging the supernatant from centrifuge operation 1 at 1,000,000 xg / 60 min in centrifuge operation 2, it was possible to precipitate the high-purity silver colloid. Also, it was possible to perform concentration by re-dispersing this precipitate. Also, the particle diameter of the concentrated silver colloid was mostly the same as that of the primary particles.

