Fundamentals of Ultracentrifugal Virus Purification
Nagasaki University Graduate School of Biomedical Sciences
Molecular Epidemiology
Professor: Dr. Osamu Nakagomi
In recent years, in virus research, it has become standard practice to purify and analyze genomes and identify viruses from samples using commercial kits. Because it has been established by prior research which viruses have which genomes, virus identification is possible even as-is in a mixed state. However, in order to investigate which genomes are present in virus particles in order to make a detailed investigation of the nature of a virus, it is first necessary to refine the virus particles to a high level of purity. Our company is also receiving an increasing number of inquiries asking for detailed information on ultracentrifugal virus purification, so we asked Dr. Osamu Nakagomi, a professor at Nagasaki University, who is highly knowledgeable about ultracentrifugal virus separation, about the fundamentals of ultracentrifugal virus purification.

Please tell us about your research background, professor.
My specialty is the molecular epidemiology of rotavirus. Until the 1970s, the causes of diarrhea in infants were almost entirely unknown, including whether viruses were involved or not. With the discovery of rotavirus in 1973 came the finding that rotavirus was the cause for half of the patients admitted to pediatric wards for diarrhea. This was an epochal discovery. A vaccine was completed in 2006 after many complications, and it has been in use since 2011. Currently, the question of whether to institute periodic inoculation is being studied by the Ministry of Health, Labor and Welfare.
Rotavirus is a non-enveloped virus with a genome that consists of double-stranded RNA with 11 segments (Fig. 1). Like other RNA viruses, it evolves rapidly and, because of its rich genomic diversity, infects not only humans, but also a variety of mammals and birds. In the 1980s, using genome-level molecular epidemiological methods for the first time, we discovered that animal rotavirus can be pathogenic and can infect humans. Elucidating how rotavirus evolves while it uses humans and other animals as hosts—and how infection spreads—is the core of our research. We are also investigating what changes will occur with the use of vaccines. This kind of research is in the field of molecular epidemiology, which investigates virus genomes
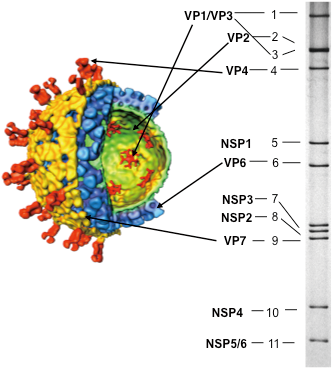
Fig. 1. Rotavirus particle structure and genome RNA Left: The intact particle has an outer shell made of red and blue proteins and has a three-layered structure, so it is called a triple-layered particle (TLP); if the outer shell is peeled away, a double-layered particle (DLP) with blue proteins remains. Right: The genome RNA of rotavirus is separated into 11 segments with characteristic patterns shown by electrophoresis. The diagram of the rotavirus particle was kindly provided by Professor Prasad of Baylor University.
Please discuss the necessity of ultracentrifugation.
When extracting a virus genome using the classical method, the virus particles must first be purified. Then the virus genome extracted from the particles is analyzed. Ultracentrifugal virus purification plays an important role in this process. Purifying the virus particles makes it possible from the start to ensure that we are dealing with the doubt that the genome is present in rotavirus particles. However, if we are dealing with the problem of determining what kind of host cell organelles or virus proteins and genomes are gathered in an infected cell, ultracentrifugation becomes necessary.
Moreover, when researching new viruses, it is necessary to investigate whether or not the genome is present in the virus particle. In such cases as well, ultracentrifugal purification is necessary. Information on the buoyant density, size, and rotavirus genome in the virus particles. Currently, analysis is nearly always performed after hastily extracting the genome without purifying the specimen. This is because the kind of genome present in the rotavirus particle is known, and it is a basic premise that if the genome (Fig. 1, right) characteristic of rotavirus is present, there is no sedimentation coefficient (Svedberg value, S value), which are involved in ultracentrifugation, is the primary foundation of virology. This basic information is called the physicochemical properties of the virus.
Please give us an outline of ultracentrifugal purification of viruses.
When purifying rotavirus from patients, we must separate cell organelles, biological macromolecules, and the like from the virus. These particles have a variety of sizes and densities. The ultracentrifugal purification of viruses utilizes gaps into which other particles do not come by using a combination of “sucrose density gradient centrifugation, which is affected by the S value of particles” and “cesium chloride density gradient equilibrium centrifugation (density equilibrium ultracentrifugation), which is affected by the buoyant density of particles in a solvent.” Fig. 2 shows the relationship between banding density (the density at which bands appear during density gradient centrifugation) and S value. The virus is located exactly in gaps between a variety of cell particles, including microsomes and ribosomes. Viruses are complexes of proteins (mostly with a buoyant density of 1.3 g/cm3) and nucleic acids (mostly with a buoyant density of 1.7 g/cm3), so the buoyant density is 1.35–1.4 g/cm3. On the other hand, the size of viruses reaches S values of 100–1,000 S. The S values of microsome fractions overlap with those of viruses (horizontal axis), but separation by density is possible (vertical axis). Thus, separation is performed using density gradient centrifugation of sucrose, cesium chloride, and the like. Virus fractions have a higher density than microsome fractions because they include nucleic acids. Moreover, the larger the virus, the larger its S value. Ultracentrifugation involves purification by combining these two parameters, so it is apparent that it is closely tied to physicochemical properties.
The centrifuge is also extremely important for improving our direct sense of these physicochemical properties. Size can also be reliably observed with an electron microscope, but if a new strain of virus is present, the sense of where the virus will fall when centrifuged is important. For instance, influenza and the rotavirus, which are of moderate size, on the order of 100 nm, will drop if spun overnight for about 10,000+ rotations using an Avanti high-speed refrigerated centrifuge. However, norovirus is on the order of 30 nm in size, so it will not drop with the Avanti centrifuge and an Optima ultracentrifuge becomes necessary. To make 30 nm particles drop with an Avanti centrifuge, purification is performed after volume is reduced using operations such as salting.
Moreover, with regard to process volume (departure sample) methods, from largest to smallest volume requirement, include: differential centrifugation (normal pelleting); cushion method; density gradient method. The cushion method allows the tube to be filled 60–70% with the sample, but the volume becomes around 10–15% for density gradient centrifugation. Thus, after ascertaining the behavioral characteristics of the particles using the density gradient method, the cushion method is used to enable a greater volume to be processed. For instance, the rotavirus particle passes through a 30% sucrose layer, but does not pass through a 70% sucrose layer, so the virus can be concentrated at the boundary between 30% and 70% sucrose layers (Fig. 3).
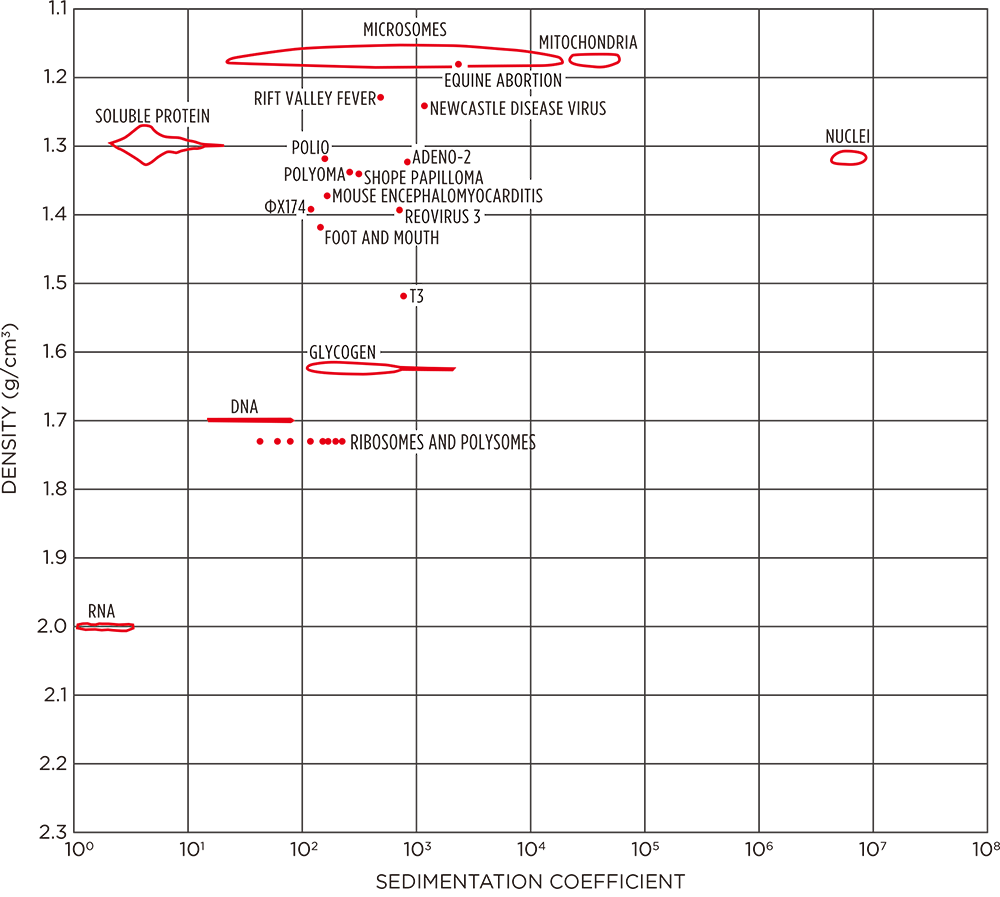
Fig. 2. Virus / organelle density and S value1 )
Please tell us about ultracentrifugal purification of a specific virus.
Fixed-angle versus swinging bucket rotors
Generally, if rotors that produce the same centrifugal force are compared, the fixed-angle rotor allows a greater volume to be processed and is easier to use. However, because top-loading swinging bucket rotors such as the SW 32 Ti allow buckets to be dropped in from above the rotor, the difference in ease of use may be eliminated. In fact, because swinging bucket rotors require a lid on the tube, it could be argued that they are in fact easier to handle. If you want to cleanly remove bands produced by density gradient centrifugation, swinging bucket rotors are preferred. Pellets leave tails on the wall surface with fixed-angle rotors, which some people may consider problematic. In particular, recently, the detection sensitivity of real-time PCR has increased, making it necessary to consider levels of contamination that could have previously been ignored.
In general, then, I think fixed-angle rotors should be selected if larger volumes are required, and swinging bucket rotors should be used if contamination is a major concern.
Sample preparation to crude centrifugation
I will explain the sample preparation step using the purification of rotavirus from a culture as an example. Because of yield relationships, if you want to extract the virus from a culture, the starting volume should be about 1 L. Thus, after a certain degree of concentration using a high-speed refrigerated centrifuge, a concentration and purification step is performed with a floor-type ultracentrifuge using a swinging bucket rotor SW 32 Ti or SW 55 Ti. In my opinion, the SW 32 Ti is a "dream rotor" because its design makes it extremely easy to use. We use this rotor in our Optima ultracentrifuge from Beckman Coulter Life Sciences. I consider our Optima a trusted lab partner, especially every time I run it overnight. I know I can always trust it to give me reliable answers the next day, and I've never been disappointed.
Fig. 3 shows our protocol for purifying rotavirus from a culture. First, to eliminate cell fragments, divide 1 L of culture into six bottles and centrifuge for 10 minutes at 15,000 x g using a fixed-angle rotor JA-14. This step can be omitted, but there will be more impurities during ultracentrifugation using the cushion method at the next stage. The more pretreatment applied, the easier and cleaner subsequent operations become. Moreover, if there is a large quantity of impurities, the virus becomes enmeshed in them, which can decrease yield.
Next, subject the supernatant with cell fragments removed by centrifugation for 14–16 hours at 30,000 x g using a JA-14 fixedangle rotor. If you are in a hurry, you can use an ultracentrifuge and process 300 mL at a time with a Type 45 Ti fixed-angle rotor. You can either rapidly centrifuge these samples at 30,000 rpm/2 hours or 40,000 rpm/1.5 hours.
Next, suspend the pellets in 26 mL of phosphate buffered saline (PBS). Deciding what concentration of pellets to use at this point requires experience and intuition. As a general rule, concentrations higher than 1 to 10 should not be used in a single step. For instance, if the starting volume is 1 L, you should not concentrate to below 100 mL; if you concentrate to, say, 10 mL, the result will be muddy. If you want to make it as easy as possible, you may end up with high concentrations, but remember this is only a guideline.

Fig. 3. Purification of rotavirus
Ultracentrifugal purification
Next, remove the impurities using the sucrose cushion method with a large-capacity SW 32 Ti Swinging Bucket Rotor. The rotavirus particles can be concentrated at the boundary between the 30% and 70% sucrose. Place 4.5 mL of 70% sucrose solution at the base of a 38.5 mL ultracentrifuge tube, layer 6.5 mL of 30% sucrose solution over this, and then layer 26 mL of the virus suspension, suspended in PBS, on top. If ultracentrifugal treatment is applied under the conditions in Fig. 3, impurities will pool in large quantities above the 30% sucrose solution and the rotavirus particles will pool between 70% and 30%. Collect this white intermediate band. This can be done in three ways: using a syringe, insert the needle into the band and extract it; collect the required band after removing the unneeded liquid above; or separate the fractions by inserting a needle from below.
In order to further increase the degree of purity, perform density gradient centrifugation using a small-capacity SW 32 Ti Swinging Bucket Rotor. Place 55% (w/v) cesium chloride solution at the base of a 5 mL ultracentrifuge tube and then layer the same quantity of 40% (w/v) cesium chloride solution over this. Density gradations are produced by layering solutions with different densities, and this procedure is often considered time-consuming. However, a density gradient is possible even with only two layers. This is a little trick. Layer 0.5–0.8 mL of virus crude fraction obtained by the cushion method (it is better to use a small quantity of the solution in this case) over the cesium chloride solution that has been layered, and perform ultracentrifugation for 16–20 hours at 257,000 x g. Sixteen hours means starting centrifugation in the evening of the previous day and collecting the next morning, but there is no need to be strict about this duration.
As you can see in Fig. 3, there are two visible bands of rotavirus. The upper band is 1.36 g/cm3 intact rotavirus (triple-layered particle: TLP) and the lower band is 1.38 g/cm3 rotavirus with the outer shell removed (double-layered particle: DLP). The virus particles contain RNA. The DLP has few proteins and the proportion of nucleic acids is higher, so it is heavier. The blue band above is empty particles consisting only of proteins with no nucleic acids and, because they are proteins, the buoyant density is around 1.3 g/cm3. In fact, when the outer part of the TLP is removed to reveal the DLP, RNA polymerase is activated. In experiments involving RNA polymerase, DLP rotavirus with the outer part removed is used.
Rotor cleaning
Important advice: because high-density cesium chloride is used, wash the rotor thoroughly with lukewarm water after use to prevent pinhole-like corrosion.

The staff of the Molecular Epidemiology Laboratory

