免疫疗法给癌症治疗带来了新希望
"Hitting the T-cell Accelerator" and Other Fast-moving Strategies

Dr. Leena Gandhi

Dr. Jill O'Donnell-Tormey

Dr. Leena Gandhi

Dr. Jill O'Donnell-Tormey
In 2017, those interested in immunotherapy research were invited to participate in two insight-filled webinars, one featuring Dr. Leena Gandhi, Director of Thoracic Medical Oncology at NYU Perlmutter Cancer Center, and the other presented by Dr. Jill O’Donnell-Tormey, CEO and Director of Scientific Affairs at the Cancer Research Institute in New York City.
Dr. Gandhi’s research focuses on early drug development for lung cancer, specifically the potential for using novel immunotherapy combinations for treatment.
Dr. O’Donnell-Tormey joined the CRI in 1987 and has served as CEO since 1993. Prior to joining CRI, she was a CRI post-doctoral fellow in the laboratory of cellular physiology and immunology at Rockefeller University.
In addition to the fact that their current roles landed them both in New York City, these two experts share a similar opinion about immunotherapy: despite many ups and downs over the past few decades, its role in developing future cancer treatments looks increasingly promising.
Stops and Starts: Immunotherapy's Early Years
“Immunotherapy has been plagued with a lot of overly hyped expectations,” says O’Donnell-Tormey, citing in particular the designation of monoclonal antibodies as a “magic bullet for cancer,” way back in 1970. Unfortunately, soon thereafter, those “magic bullets” turned out largely to be blanks.
“When that did not come to fruition,” she says, “everyone lost a lot of optimism about the field in general.”
The early history of immunotherapy was marked by starts and stops—and arguably more of the latter than the former. Following the “magic bullet” misfire of the 1970s, the first monoclonal antibody tested for lymphoma failed in the 1980s. Much later in that decade, however, interferon gamma was approved for treating melanoma. In 1991, the first tumor antigen was identified, which provided some researchers with renewed hope that we could indeed harness the power of the human immune system to directly attack cancer cells.
While the first monoclonal antibody to achieve success (Rituximab) was finally approved in 1997, the 1990s also saw the failure of a number of cancer vaccines, which many thought might prove to be a powerful new strategy for cancer prevention. With the notable exception of the human papilloma virus vaccine, with its demonstrated ability to prevent HPV-related cancers, success stories in the field of immunotherapy were still few and far between for many years.
Fast forward to the second decade of the 21st century, and immunotherapy has again become, in O’Donnell-Tormey’s words, “the new darling of oncology.”
"In 1991, the first tumor antigen was identified … which renewed hope we could indeed harness the power of the human immune system."
In 2011, an anti-CTLA-4 checkpoint blockade was approved for metastatic melanoma—representing the first treatment to have an impact on overall survival rates for late-stage melanoma. Since then, the FDA has approved 13 different immunotherapies for 12 types of cancer.
“Science magazine named (immunotherapy) Breakthrough of the Year in 2013, and it was ASCO's top advance in 2016,” O’Donnell-Tormey notes, referring to the American Society of Clinical Oncology. “At ASCO's recent meeting in June, it was the topic of many talks—a big change from 10 years ago, when immunotherapy was off to the side in (a) small conference room on the last day, (and) no one attended.”
The irony of labelling immunotherapy a “breakthrough” in 2013 isn’t lost on Dr. Gandhi, who echoes O’Donnell-Tormey’s reminder that immunotherapy is in no way a new concept in cancer treatment. Dr. Gandhi, however, does draw a distinction between active and passive immunotherapy—the latter, in particular, now generating a lot of interest among her and her fellow oncologists.
"’Active’ immunotherapy, or trying to stimulate a response against tumors, includes cytokine-based therapies (and) cancer vaccines, which have also been an area of active interest for many years in many different tumor types,” says Dr. Gandhi.
"’Passive’ immunotherapy, on the other hand, is the opposite—blocking inhibition against the immune response at the tumor or (its) microenvironment. (This) is a newer concept in oncology treatment, and started with the development of anti-CTLA-4 antibodies, and more recently, has transformed the field with development of anti-PD-1 or PD-L1 antibodies.”
These specific antibodies create what are called checkpoint blockades, a tool that has clearly “revved up” recent enthusiasm about new immunotherapies for cancer.
Attention: Checkpoint Ahead
How do checkpoint blockades work? O’Donnell-Tormey likes to compare the process to that of driving a car.
She explains: “For a T-cell to become activated—to become an effector cell that can destroy a cancer cell—it requires two signals. First, the T-cell receptor needs to ‘see’ the marker that identifies (a) major histocompatibility complex (MHC) antigen presented by something like a dendritic cell. If that signal happens by itself, the T-cell fails to activate and becomes dormant and dies. What’s needed is a second signal, a marker called CD28 on the T-cell (that) binds to a B7 marker on the antigen-presenting cell (APC). When the two signals happen simultaneously you get T-cell activation.
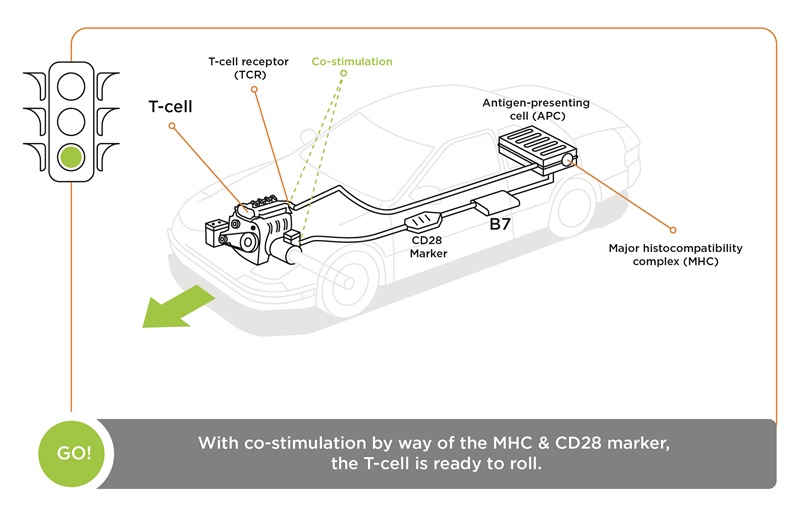
“The immune system has natural checks and balances,” she continues. “And one of these is CTLA-4, which we call the ‘brake.’ As the two signals are activating the T-cell, it starts to produce CTLA-4 on its surface, (which) can out-compete CD28 binding to the B7 molecule. When CTLA-4 binds to B7, that sends a stop signal. It stops the T-cell from becoming activated, and it becomes dormant.
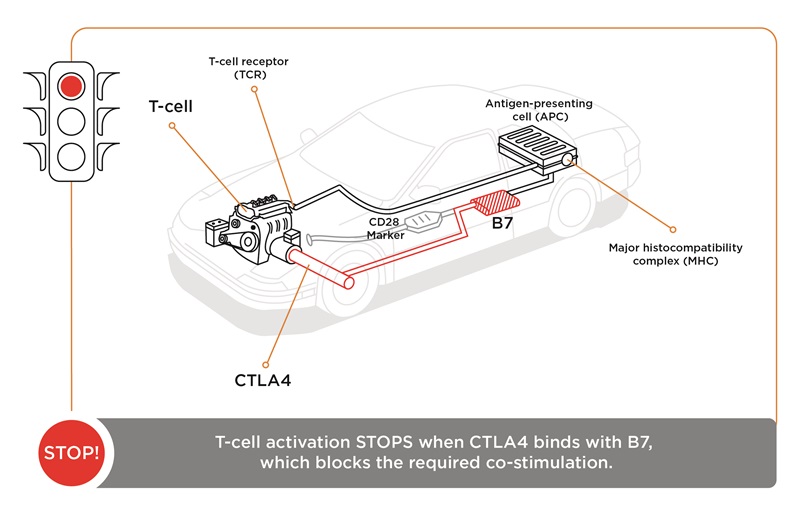
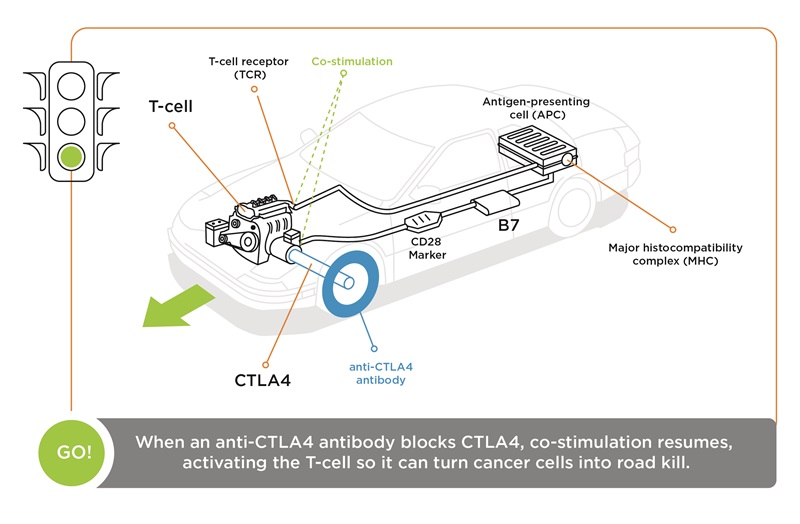
“(With) checkpoint blockades, an antibody (is) created that binds to the ‘brake’—the CTLA-4 molecule—which can no longer bind to B7, so the T-cell stays in an activated state. Simply stated, an antibody against CTLA-4 ‘takes the brakes off’ the immune system.
“(If) we think of the immune system as a car, the first signal, the T-cell receptor antigen-MHC binding, is similar to putting the key into the ignition. (But) the car doesn't move until you put your foot on the gas. And the immune system’s gas pedal is CD28 and B7. To stop the car, you need to put your foot on the brake, and the brake is CTLA-4 and B7. So, if you disrupt the CTLA-4 binding to B7, the T-cell keeps working, so the car keeps running.”
Other checkpoint blockades, such as those targeting programmed cell death protein 1 (PD-1) and one of its ligands, PD-L1, can also fight back when cancer cells co-opt the immune system’s natural checks and balances.
Tumor cells produce PD-L1, which binds to a PD-1 receptor on the T-cell, thereby essentially shutting off the immune system by enabling tumor cells to become “invisible.” By creating antibodies that bind to either PD-L1 or PD-1 on the T-cell, we can stop that interaction and allow T-cells to “see” and destroy the tumor cells.
To date, the FDA has approved six checkpoint blockades, one against anti-CTLA-4 and several against either PD-1 or PD-L1. They’ve been approved for seven different cancer types—and not only for late-stage cancers, but also for frontline treatment of melanoma, bladder cancer and non-small cell lung cancer.
“There’s a correlation,” O’Donnell-Tormey says, “between cancers that have higher mutation rates, such as melanoma, having a higher response rate to checkpoint blockades—as high as 40-45%. (But) some cancer cells, such as prostate or uterine sarcoma, haven’t shown any response rate to checkpoint blockades.
“And then you see a variability between 10% up to 40%, these are impressive responses—but it's also telling us only a subset of patients with various cancer types actually respond.”
“Simply stated, an antibody against CTLA-4 ‘takes the brakes off’ the immune system.”
That problem is something with which Dr. Gandhi can readily relate.
“There’s an ongoing controversy about the predictive value of PD-L1 expression on tumor cell in terms of being able to generate (a) response,” Gandhi says. “We know only a subset of patients are getting a long-term benefit, but it's still an open question (about) the best way to identify those patients.
“We do know from a large body of work to evaluate somatic mutation frequencies in different tumor types, that tumor types that have seen the most benefits from PD-1 and PD-L1 inhibitors have something in common. They all have the highest mutational burdens. The first approvals for PD-1 therapy came in melanoma, the second in lung cancer and the third in bladder cancer—which are all tumor types with the highest overall mutational burden, (and) that makes sense.
“If there's a lot of mutational change in tumor cells at the DNA level, we would expect the tumor (to be) more immunogenic and recognizable by the immune system.”
Though there’s still much that scientists need to learn about checkpoint blockade therapy, many expect it to eventually become a first-line treatment for many types of cancer. In fact, the PD-1 checkpoint blockade can already be a better choice than chemotherapy or radiation because it has demonstrated similar efficacy with less-severe side effects.
And while CTLA-4 and PD-1 are currently the focus of most immunotherapy studies, researchers expect to uncover additional checkpoints that will eventually share that spotlight. For now, they know that multiple checkpoints appear to be co-expressed with PD-L1 and PD-1 in tumors. Consequently, some of these molecules will soon be tested in clinical trials, many in combination with anti-PD-1 or anti-PD-L1 blockades.
The Promise of Immunotherapy: No Longer a Chimera?
In addition to checkpoint blockades—a therapy recently spotlighted in mainstream media because of its successful use in treating former President Jimmy Carter’s melanoma—another encouraging immunotherapy tool has recently taken center stage. Chimeric antigen receptor (CAR)T-cells are genetically engineered immune cells derived from, and then returned to, the patient’s own bloodstream.
Specifically, a patient’s T-cells are collected, then sent to a lab or drug manufacturing facility where they’re genetically engineered to produce chimeric antigen receptors (CARs) on their surface. CARs are proteins that enable T-cells to recognize a specific antigen on the tumor cells being targeted for that individual patient.
Reengineered cells are then grown in the lab, with each “batch” of (millions of) cells frozen until there’s a sufficient amount for treatment. They are then sent to the hospital or treatment center where they’re infused into the patient. Many of these patients undergo a brief course of chemotherapy before they receive the infusion of CAR T-cells.
After infusion, CAR T-cells multiply in number, creating a virtual army of “attacker” cells that recognize and kill cancerous cells that have the targeted antigen on their surface.
After winning the initial war, CAR T-cells also guard against recurrence of the patient’s cancer, because CAR T-cells can remain in the body long after the infusion. Consequently, CAR-T-cell therapy frequently results in long-term remissions.
“Response rates are truly remarkable,” O’Donnell-Tormey says. “For leukemia, we’re seeing response rates that range from 60% to 100%, depending on the type of leukemia. Some side effects, unfortunately, have been severe, and the incidence of adverse events is significant.”
Tempering excitement about CAR-T-cell therapy has been more than just the challenges presented by its side effects.
“The cost of CAR-T-cell therapy is very high,” says Dr. Gandhi, “because it has to be personalized for every individual. Don’t get me wrong: all of these therapies are expensive. The cost for PD-1 inhibitors is astronomical and, really, unheard of in the world of oncology. But CAR-T-cell therapy goes one step further.”
In 2017, the FDA approved the first CAR-T-cell therapy—tisagenlecleucel—for pediatric and young adult patients with B-cell precursor acute lymphoblastic leukemia. At an estimated cost of more than $500,000 per patient, it was not surprising that this new form of therapy was greeted with a combination of excitement and controversy.
For some clinicians, the current costs for treating this form of leukemia, including, for example, the cost of an autologous bone marrow transplant (typically $350,000), help put the value of tisagenlecleucel in perspective. With an entire lifetime hanging in the balance, leukemia patients represent a unique cohort for comparing the cost/benefit ratio of novel and complex treatments such as CAR-T-cell therapy.
But in lieu of diving into the middle of a cost/benefit/value debate, Drs. Gandhi and O’Donnell-Tormey would rather focus on another promising strategy for using immunotherapy: combination therapy.
Additive Impact: The Power of Combination Therapy
Dr. Gandhi can point to many studies currently underway that are evaluating immunotherapy combinations.
“The one that’s been most evaluated—and led to changes in treatment for melanoma at least—is the combination of PD-1 inhibition with CTLA-4 inhibition,” she says.
“This was the area where they first evaluated combination treatments and demonstrated in multiple large-scale studies that combination therapy is superior to monotherapy.
“This study was meaningful (because) it showed there was an overall improvement with combination therapy compared to either monotherapy. But for PD-L1-positive patients, there was really no difference between combination and monotherapy. The greatest difference (was) in those who were PD-L1 negative.
“And this is important for two reasons: one, we don't want to give patients more therapies—and more potential toxicities—if we don't have to. But two, we want to be able to provide benefits to patients who are unlikely—or less likely—to get (them) with anti-PD-1 or PD-L1 therapy on its own.”
“Most clinical trials pertaining to lung cancer now are focused on immunotherapy combinations…”
Dr. O’Donnell-Tormey concurs with Dr. Gandhi’s prognosis for the ongoing development of novel combination therapies, citing the inherently synergistic nature of immunotherapy.
“It can be combined not only with different immunotherapies but also with radiation and chemotherapy,” she says. “And it can be very targeted. If you can figure out exactly how the marker on a cancer cell can be ‘seen’ by the immune system, you can target that response to go directly for the cancer and leave aside normal cells, which is obviously one of the great hopes for any cancer treatment.
 “By combining checkpoint blockades or CAR T-cells with other treatments or with themselves, we can get more patients (who) are responding for longer periods of time. Combinations make sense, because to get an effective immune response against cancer takes a variety of steps. It's not a one-step process.”
“By combining checkpoint blockades or CAR T-cells with other treatments or with themselves, we can get more patients (who) are responding for longer periods of time. Combinations make sense, because to get an effective immune response against cancer takes a variety of steps. It's not a one-step process.”
Dr. Gandhi agrees, here again speaking directly from experience. “Studying this in lung cancer,” she says, “there was a keen awareness that one difficulty with this combination is that toxicity is also greater. But one way of mitigating the increased toxicity with combinations might be to change the dosing schedule and dosing combination.”
As Dr. O’Donnell-Tormey mentioned, combining specific immunotherapies is only one possible strategy for identifying new and effective cancer treatments. Of greater interest to Dr. Gandhi is whether patients who’ve been exposed to chemotherapy, radiation therapy or other targeted therapy might be either less or more likely to respond to subsequent immunotherapy.
“I think that's a critical area of research,” she says, “because it's likely that most patients will be exposed to these other therapies. These tools for treating cancer are not going to go away.
“In fact, I’d say most clinical trials pertaining to lung cancer now are focused on immunotherapy combinations—and the potential combination partners are many.
“We know chemotherapy is immune-suppressive, meaning it should deplete derived suppressor cells. Theoretically, it should make a tumor more immunogenic. The same is true for radiation.”
And what of the idea that immunotherapy as a first-line treatment might help ensure greater success with subsequent use of traditional tools such as chemotherapy? Some researchers hypothesize that immunotherapy could, in essence, “prime” a chemotherapy response.
“I don’t think that's just pure speculation,” Gandhi says. “(But) I don't think we know that as yet.”
One thing seems to be certain, however: a combination of therapies that includes immunotherapeutic tools might one day outpace monotherapy to become a standard treatment protocol for a variety of cancers. Before that can happen, researchers continue to face the formidable challenge of identifying which patients can benefit most from a particular combination therapy—with minimum impact from the compounded side effects combined therapies can produce.
Immunotherapy in the Coming Decades: Will Research Accelerate New Discoveries?
 Dr. O’Donnell-Tormey and Dr. Gandhi are both unapologetically optimistic about the future of immunotherapy, regardless of its history of proceeding three miles ahead, then one mile back.
Dr. O’Donnell-Tormey and Dr. Gandhi are both unapologetically optimistic about the future of immunotherapy, regardless of its history of proceeding three miles ahead, then one mile back.
“First and foremost, (immunotherapy) is universal and powerful,” says O’Donnell-Tormey. “And it could prove effective for all types of cancers. We know it can change cancer into a manageable disease, if not cure it. It's also adaptable. The immune system is always ready to protect us against unknown challenges, so as cancers mutate, our immune system can adapt to that and still mount a defense.
“We've never before had such a large number of patients who have positively responded to immunotherapy.”
“We’ve also seen over the past few years, not only in animal models but in patients, that the responses of immunotherapy can be durable, perhaps because the immune system has memory. This innate ability is one of the hallmarks of (our) immune system, and one of the reasons why it’s such an appealing way to potentially treat cancer.”
For Dr. Gandhi, looking down the road at where immunotherapy can take us in the years ahead is encouraging based on the results she’s already seen in the rearview mirror.
“We’re seeing some lung cancer patients, close to 20%, who are long-term survivors with immunotherapy,” she says. “And these are patients with metastatic disease, who otherwise would have been expected to live no longer than a year.
“At three years, we see a 27% survival rate—unheard of in lung cancer, where a two-year survival rate is typically 11%, and at three years, almost zero. So for these patients, and it is still a subset, they’re getting long-term benefits we don't see with chemotherapy, or with many targeted therapies either.”
Few would argue that the lessons of the past, combined with recent successes, bode well for the ongoing progress of immunotherapy in the field of oncology. There may be no “magic bullets,” but recent advancements suggest there’s a high probability we can eventually develop an armory of effective immunotherapeutic weaponry for managing or defeating cancer.
“Having been in this field for a very long time, I believe we’re at a unique position,” says O’Donnell-Tormey.
“We've never before had such a large number of patients who have positively responded to immunotherapy. Unfortunately, a lot have not responded—but that gives us a platform where we can begin to ask questions we've never been able to before.
“We've had this big leap—from people not believing the immune system could be used as a cancer treatment, to having a big bandwagon everybody is jumping on, saying ‘immunotherapy is revolutionizing cancer treatment,’ which I believe it is.
“In the future, I think immunotherapy—or, more accurately, precision immunotherapy—will be a backbone of cancer treatment.”
“At three years, we see a 27% survival rate—unheard of in lung cancer, where a two-year survival rate is typically 11%...”
Dr. Gandhi adds: “It's important to remember that we want to do better for all patients, and if we know there are ways to stimulate an immune response, and we know some of the mechanisms that can impair it, we, theoretically, should be able to overcome those and make tumors more immunogenic and more recognizable (to) the immune system. And that's where a lot of (our) current efforts are heading.
“And if we do this well with metastatic disease, obviously we would want to move all of these things earlier in the development of cancer. Some studies are being planned to look at whether we can treat intrapulmonary nodules—things that are not cancer yet—with immunotherapies to help prevent them from becoming (cancer).”
Not surprisingly, both doctors agree that ultimately winning a race against cancer in the coming years will not be unlike it has been for the past 50 years. It will continue to require a significant amount of basic and transactional research—as well as ongoing collaboration and good old-fashioned innovation.
And although the journey will no doubt be long, the wind is now at our backs and the road ahead looks clearer than ever before.
For more information, watch this webinar on the recent advances in immunotherapy presented by Dr. Gandhi.

