An Overview of Biopharmaceutical Production and QC
Introduction
Biopharmaceutical drug production is subject to strict quality guidelines and regulations. One major concern for GMP environments is following 21 CFR Part 11 guidelines for data integrity. A GMP Manufacturing facility must meet many standards, and creating an audit trail through the production process is essential for adhering to 21 CFR Part 11. The FDA has issued guidelines for data integrity using the acronym ALCOA+. Data must be:
- Attributable, linked to an individual associate and instrument.
- Legible, presented in a clear and standard format.
- Contemporaneous, created alongside data generation.
- Original, not transcribed or photocopied.
- Accurate, unaltered and avoiding manual calculation or data entry.
- +, ensuring data is complete and available after the recording instrument is no longer used.
Adhering to these guidelines at every step of the biopharmaceutical production workflow is key for avoiding a costly process audit. Instruments from Beckman Coulter Life Sciences offer integrated and automated ALCOA+ adherence solutions for every step of this workflow.
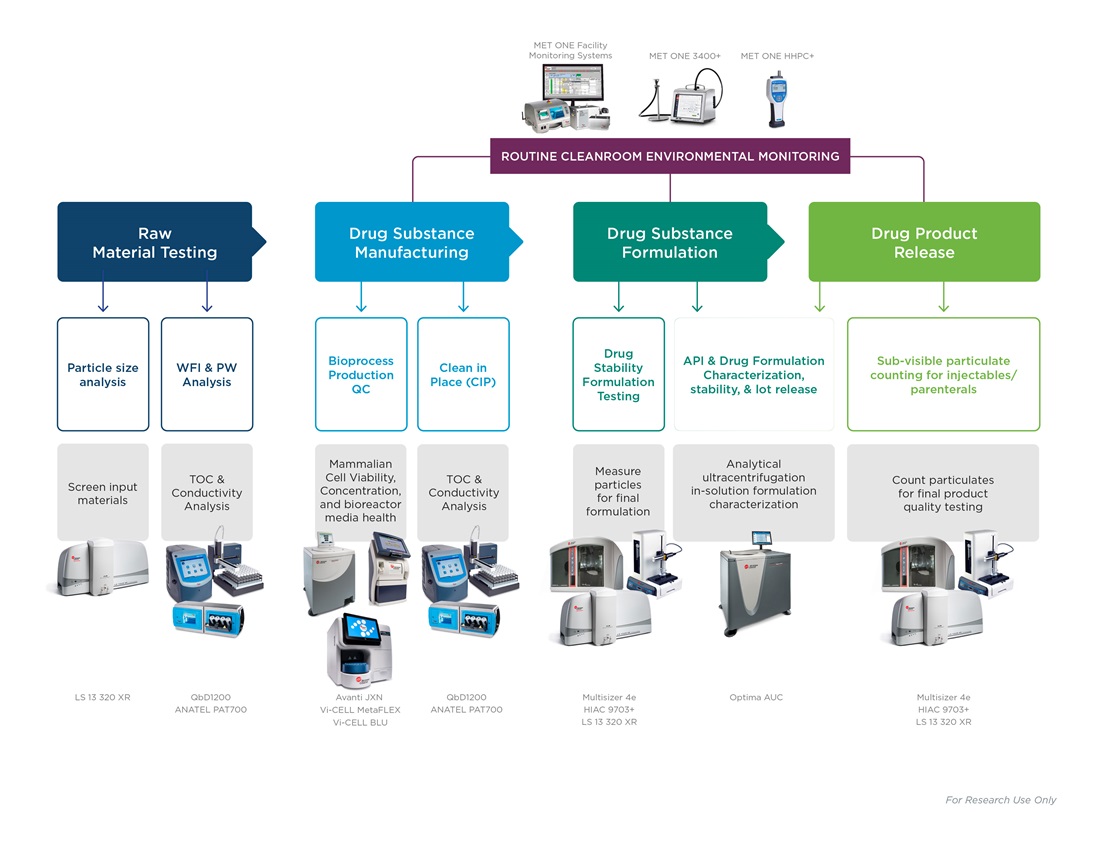
Water Quality Monitoring
Availability of purified water and water for injection is a prerequisite for drug production workflows and routine facility clean-in-place protocols. Water is the most important raw material in production. Incoming water used in all aspects of a workflow must be tested for total organic carbon and conductivity, ranging across:
- Cell culture media
- Clean-in-place washes and rinses
- Water for Injection for final formulation
Any potential contaminants in incoming water risks compromising the entire workflow downstream. Water testing may be performed constantly with on-line equipment for recirculating facility loops, or at a single point with laboratory analyzers for a specific procedure or quality measurement.
Environmental Monitoring
Pharmaceutical production is highly regulated, taking place in cleanrooms and engineered spaces with little to no airborne particulate contamination. Production spaces and facilities must be constantly evaluated for airborne particulate matter, with microbes brought in by human associates being of particular concern. Often, monitoring equipment is bulky, subject to human error or incorrect usage. Lightweight air counters and preprogrammed SOPs that direct associates to monitoring points help to keep cleanrooms compliant.
Raw Materials Testing
Starting ingredients and excipients for production are evaluated for consistency and suitability. Dry powders, suspensions, or emulsified particulate matter are subject to strict quality control before production workflows begin. Ingredients must be of correct and precise size distributions to facilitate consistent final products and proper administration of the active ingredient to patients. Automatic pass/fail calculations and preloaded, push-button SOPs take guesswork out of raw material suitability.
Bioproduction
Active ingredients of drug products are expressed by cells grown in bioreactors. Culture conditions and media must be closely monitored, as nutrient imbalances or maturing cultures could affect product yields. Cultures in post-exponential growth phase or that have accumulated too high of a biomass may begin to undergo apoptosis, compromising production. Cell count, viability and activation state are key parameters to measure in bioproduction.
Drug Formulation
Active ingredients harvested from cell cultures are combined with excipient ingredients, such as stabilizers or preservatives, to create a stable solution for administration to a patient. Testing for particulate matter or protein aggregation at this step is important for product consistency between batches and production sites.
Final Testing
Before release to shipping, formulated products are evaluated for particulate contamination and overall quality in compliance with local Pharmacopoeia guidelines such as USP <787>. Drug products for injection must be evaluated for sub-visible and visible particle concentration and volume. Therapeutic protein particles are small enough to not impact a test result; however, protein aggregates are undesirable and indicative of poor product quality. After passing all quality checks, the drug is shipped.
Conclusion
Adhering to regulations and guidelines for pharmaceutical production and quality control doesn’t have to be a headache. Instruments from Beckman Coulter Life Sciences are designed to create data and export original electronic records securely in parallel with quality control measurements. There is an evergreen requirement for data generation and management in production facilities. Ensure your electronic data trail is robust with instruments from Beckman Coulter Life Sciences.

