Cell-Line Engeneering
A Check-up Before You Scale Up
Bioproduction is a booming industry, with more therapeutics being produced in cell culture than ever before. With increased workflow flexibility, decreased variability, and high product yields, cell-line engineering is here to stay. But before you take your bioproduction to the next level, you’ll need to know a bit more about your product, your cells, and their health. It’s time for a cell-line engineering check-up!
 Transfection
Transfection
There are several transfection methods available, including electroporation, lipofection, calcium phosphate, polymerbased methods, and viral transduction. Choose one that is optimized for your cell line, vector size, and throughput. Screen for successful, stable transfectants before moving on to optimize your media and culture conditions.
Selection
Select for cells that have taken up the plasmid by applying selective pressure, either an antibiotic or medium lacking an essential amino acid. Cells that survive will have incorporated both the gene of interest and the selection marker.
 Expression Vector
Expression Vector
For optimal expression of your product, choose an expression vector with proven success in your chosen cell line. Ensure that you include the genes encoding your product, strong promoters and enhancers, selection marker(s), and the required equipment for plasmid replication in your cell line of choice.
 Cell Health
Cell Health
Bioproduction can be stressful for cells, so monitoring cell health throughout the process can help prevent unexpected protein modifications or off-target products. Two common ways to monitor cell health during bioproduction are:
Morphology tracking: Follows changes in cell phenotype throughout bioproduction
Electrochemical tracking: Measures the extracellular changes created by cell activity
Influences
What factors can influence cell health during bioproduction?
- Dissolved CO2
- pH levels
- Dissolved O2
- Temperature
- Agitation rate
- Other cellular functions
 Cell Volume
Cell Volume
The volume of a cell changes throughout protein expression, so cell-volume analysis can provide information on transfection efficiency. One of the most accepted methods to measure cell volume is using the Coulter Principle.
 Cell Viability
Cell Viability
Cell-viability analysis is essential for bioproduction workflows, since dead or dying cells don’t only stop producing, but they may introduce proteolytic breakdown products into the culture. For optimal cellviability analysis during bioproduction, trypan blue dye exclusion is the gold standard method, although other methods, like flow cytometry, can be used to determine the type of cell death.
Documenting cell death
Cell death is not a one-size-fits-all process. There are several different paths to cell death, including necrosis, apoptosis, mitotic catastrophe, or failure of autophagy. Knowing which is impacting your culture can help you with preventing the same fate in future batches.
Scale UP vs. Scale OUT
Which one is right for my product? Scale up involves increasing the batch size of your culture, with the end result of increasing the amount of product derived from each batch. Mass-produced drugs are scaled up.
Scale up involves increasing the batch size of your culture, with the end result of increasing the amount of product derived from each batch. Mass-produced drugs are scaled up.
 Scale out refers to the process of production on a patient-bypatient basis. Scale out often refers to the bioproduction of autologous cells for cell therapy.
Scale out refers to the process of production on a patient-bypatient basis. Scale out often refers to the bioproduction of autologous cells for cell therapy.
Products for Cell Counting
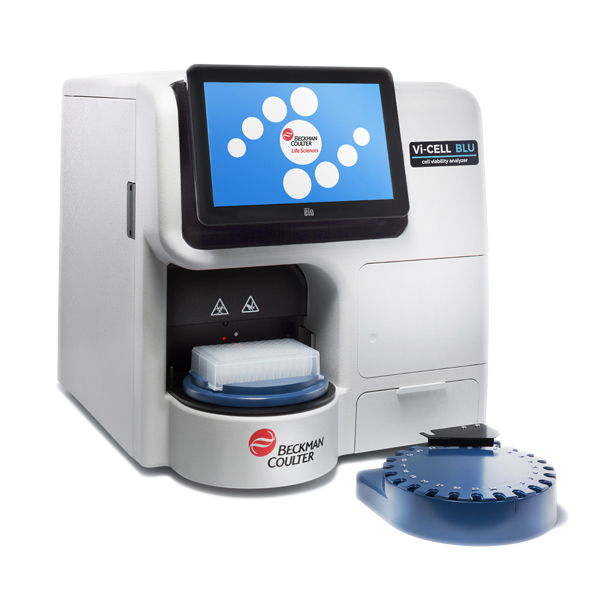
Vi-CELL BLU
Use the Vi-CELL BLU for fast, small volume viability analysis
Biomek iSeries
Use the Biomek Automated Workstation for liquid handling and automation

