Different looks, different sizes: assessing the diversity of EVs
Extracellular vesicles (EVs) are heterogeneous populations of nano- to micro-sized vesicles (30-500 nm), contain an intraluminal region, and are physiologically produced by cells, bacteria, and other living entities. They are released by budding of the plasma membrane (the large vesicles) or released from the endosomes (the smaller vesicles). EVs are defined by size (30-500 nm) and by the contents of their intraluminal regions. EVs can travel in all parts of the body and enter cells to deliver their content. EVs were discovered decades ago, but their role in physiology and disease has only recently emerged1. Currently, the study of EVs aims to
- understand their heterogeneity and involvement in pathological conditions and
- use them as cargo for drug/compound delivery as therapeutics
As such the field of EVs could bring significant scientific knowledge and contribute to medical advances in the future.
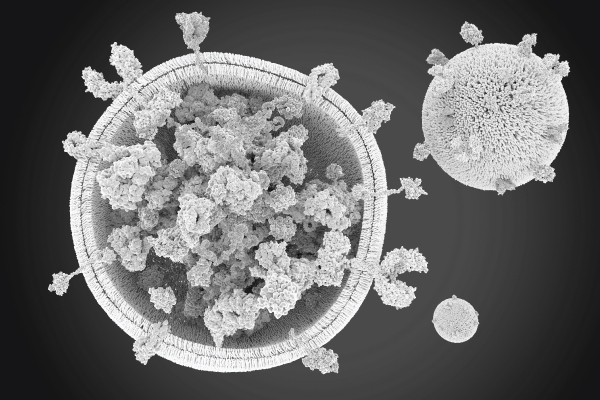
EVs are heterogeneous 2 and differ in size and content ( Figure 1). This heterogeneity arises from the different cellular origins, physiological states, and stimuli that influence EV release. The precise characterization of EVs by size and content is critical to understand their heterogeneity and comprehensively evaluate their biological functions, diagnostic potential, and therapeutic applications6. In other words, studying the heterogeneity of EVs enables researchers to better understand their function:
- Biological roles - EVs derived from different cell types, or released under different conditions, may have distinct cargo profiles and biological activities. By characterizing and comparing the heterogeneity of EVs, specific subsets of EVs could be identified that play key roles in intercellular communication, immune modulation, or disease progression.
- Diagnostic potential - EVs released by diseased cells, such as cancer cells, carry specific molecular signatures that can serve as disease biomarkers. By understanding the heterogeneity of EVs, more precise and sensitive diagnostic assays can be developed by targeting specific EV subpopulations carrying disease- specific cargo.
- Therapeutic applications - EVs can be utilized as natural carriers of therapeutic cargo, such as drugs, nucleic acids, or proteins. Understanding their heterogeneity can help to optimize their isolation, engineering, and cargo loading strategies. Additionally, the identification of specific EV subpopulations with desirable properties, such as immunomodulatory or regenerative capabilities, can expand their therapeutic potential.

Figure 1. Illustration of the variety of size and composition of EVs. The size of EVs varies from approximately 30 nm to 500 nm. Additionally, the markers expressed on the surface of EVs as well as the internal composition is very diverse. This is influenced by the cell of origin and its activation status.
Tools to study the heterogeneity of EVs
There are different tools used to determine the distribution of EVs in a sample3, the most popular being nanoparticle tracking analysis (NTA). This method is being widely used as a quality control method after isolation of EVs; however, it provides data in bulk and only provides information on the size of EVs. Content characterization is critical to understand the heterogeneity of an EV population. Similar to cells, they can be characterized using several techniques, including genomics (PCR, sequencing), proteomics (western-blot, ELISA, flow cytometry), lipidomics (chromatography, mass spectrometry), and other methods that identify and quantify their components 4,5. While these methods have proven their utility, they do not enable differentiation between proteins on the surface of EVs – critical for their identification - and those in their luminal space. Additionally, and similarly to NTA, they provide data in bulk, therefore do not allow detailed characterization of the individual EVs in the population. The single characterization of EVs can be performed using imaging methods or flow cytometry. While the first provides a visual of the EVs it is limiting because of its very low throughput and poor representation of heterogeneity.
On the other hand, flow cytometry enables researchers to characterize EVs one at a times12. Researchers have identified common markers expressed by a majority of EVs, including CD9, CD63, Tsg101, CD81, ALIX, and HSP70. While this has been often validated by western blotting or other time-consuming and bulk methods with varying accuracy, flow cytometry can offer a rapid, cost- effective and precise measure of the size, number and profile of EVs. However, this is not achieved using any cytometer and requires some specific preparation. In fact, most cytometers are not designed to detect such small particles. Concerns exist about identifying a reliably accurate method for assessing EV size and reporting on the data, and recent guidelines are leading scientists toward some consensus. In recent years, scientists have reported the ability of flow cytometers with a particular configuration (measuring side scatter via violet laser excitation) to detect particles smaller than 1 µm. Nowadays, a few flow cytometry solutions have been reported to detect EVs with various lower limits of detection (LLOD). This is an important feature of cytometers to consider, as most cytometers have an LLOD of ~ ~ 100/200 nm, while EVs below 200 nm may represent more than 50% of the population of EVs. Hence, it is of a great advantage to be able to detect the smaller particles. This avoids the exclusion by size of EVs that could have a biological importance in the research setting explored. Can we map correctly the immune system by excluding the smaller white blood cells (T, B and NK cells)? Not really. The same is likely to apply to extracellular vesicles, as no study has proved that the smaller EVs can be excluded from any preparation without affecting the potential biomarker discovery. By deciphering the complexity of EVs, it becomes possible to develop tools for early disease detection, monitoring treatment response, and even prognosis (Figure 2). The advancement of technologies enabling single-EV analysis, such as flow cytometry, will further facilitate research in this field
Perspective on EVs: breaking the barrier of translational research
EVs have been studied in various pathological conditions, including neurodegenerative diseases, cardiovascular diseases, cancer, and trauma 6-10. Understanding the contribution of EVs to a specific disease is quite a complex and lengthy task due to the amount of data needed to get a full picture. However, accumulating evidence shows EVs as a novel cellular communication channel, playing a significant role in disease processes11. This is largely attributed to the fact that EVs are naturally secreted by all cell types, and any alterations in cellular function can be reflected in the composition and quantity of EVs released. Additionally, EVs have shown potential as diagnostic and prognostic biomarkers in various diseases, including cancer, neurodegenerative disorders, and cardiovascular diseases.

Figure 2. EVs are enriched in body fluids which enables the non-invasive capture and analysis of EVs This enables to develop tools for diagnosis and prognosis prediction. EVs could also be used for monitoring therapeutic responses and predicting disease outcomes.

Figure 3. Flow cytometry principles. Particles in suspension are pushed via the sheath fluid through the system. One by one, they are excited by a light source (laser) which will enable assessment of the characteristics of the particle in terms of size, using the side scatter in the violet laser (V-SSC). While forward side scatter (FSC) is routinely used to characterize cells by size, only V-SSC enables a lower limit of detection for small particles. The light emitted is detected and converted into an electronic signal that is displayed in flow cytometry plots for analysis. Nowadays, scientists are including fluorescent probes and antibodies to further characterize EVs.
Although research is ongoing to optimize the characterization and manufacturing of EVs, several clinical trials have already been conducted to explore their therapeutic potential. One notable example is the use of mesenchymal stem cell (MSC)-derived EVs in various conditions, including Acute Respiratory Distress Syndrome, Solid Organ Transplant, Crohn’s Disease, Ulcerative Colitis, burn wounds, Dystrophic Epidermolysis Bullosa Wounds, Bronchopulmonary Dysplasia in neonates, periodontitis, severe infection, Multiple Organ Dysfunction Syndrome, and others up to more than 30 different clinical trials. This example illustrates the diverse range of therapeutic applications where EVs show promise. While most of these trials are in the early phases (I/II), their increasing number indicates a growing interest in EV-based therapies.
In addition to MSC-derived EVs, there are several clinical trials exploring EVs derived from other sources, including EVs from adipose tissue, macrophages, urine, cardiomyocytes, induced pluripotent stem cells (iPSC), neuronal stem cells,primary tumors, milk, liver stem cells, and more 11. The utilization of EVs from these various sources highlights the broad potential for EV-based therapeutics 13.
To design more optimized and targeted approaches for therapeutic purposes, a better understanding of the heterogeneity of EVs is crucial, as a specific type of EV may be more appropriate for optimal results. The ability to better resolve EVs by flow cytometry using different scatter information such as side scatter with the violet laser (Figure 3), will help to better characterize EVs and their role in disease and potential therapeutic applications.
Refrences
- van Niel et al. (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228.
- Lässer et al. (2018). Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med 60:1–14.
- Welsh et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracell Vesicles. 2024 Feb;13(2):e12404.
- Zhang H, et al. (2018) Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol 20:332–343.
- van de Wakker et al. Extracellular Vesicle Heterogeneity and Its Impact for Regenerative Medicine Applications. Pharmacol Rev. 2023 Sep;75(5):1043-1061.
- Bonafede et al. (2017) ALS pathogenesis and therapeutic approaches: the role of mesenchymal stem cells and extracellular vesicles. Front Cell Neurosci 11:80.
- Inamdar et al. (2017) Emerging applications of exosomes in cancer therapeutics and diagnostics. Bioeng Transl Med 2:70–80
- Willms et al. (2018) Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol 9:738.
- Kirkham et al. (2022) MSC-derived extracellular vesicles in preclinical animal models of bone injury: a systematic review and meta-analysis. Stem Cell Rev Rep 18:1054–1066
- Maring et al. (2019) Cardiac progenitor cell-derived extracellular vesicles reduce infarct size and associate with increased cardiovascular cell proliferation. J Cardiovasc Transl Res 12:5–17.
- Kumar et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig Transduct Target Ther 9, 27 (2024).
- Brittain et al. A Novel Semiconductor-Based Flow Cytometer with Enhanced Light-Scatter Sensitivity for the Analysis of Biological Nanoparticles. Sci Rep 9, 16039 (2019).
- Po Yee Lui et al. Practical Considerations for Translating Mesenchymal Stromal Cell-Derived Extracellular Vesicles from Bench to Bed. Pharmaceutics 2022, 14(8), 1684
Interested in learning how to advance your EV research?
Whether you have questions or are interested in a demo, we're here to help.