Automation of protein A ELISA Assays using Biomek i7 hybrid workstation
Francis O Enane, Bhagya WijayawardenaBeckman Coulter Life Sciences, Indianapolis IN
Abstract
Monitoring impurities ensures successful development of therapeutics such as monoclonal antibodies. A common method of impurity pull-down is Protein A purification. However, such purification introduces a risk for protein A cross contamination in the final drug product. Thus, methods for detection of protein A are essential for production of pure drug products destined for regulatory approval. The most frequently used methods are immunoassay designed to detect low level protein A impurities (e.g., 100pg/mL). Automation of existing protein A purification assays can enhance productivity by increasing throughput, provide analyst walk-away time and decrease human error. This application note describes an automated method by using the Beckman Coulter Biomek i7 hybrid workstation to perform protein A ELISA assay using a kit from Cygnus Technologies, Inc. The automated ELISA was designed to achieve high sample throughput, flexibility in sample handling, and consistent high-quality data. Our results indicate successful automation of this assay, which can be directly applied to other ELISA protocols/kits.
Introduction
The recent increase in the development of biopharmaceuticals and the demand for products of high quality to undergo regulatory approvals contribute to a rise in the number of analytical samples.¹ Large scale demand for process analysis consequently reduces productivity. A potential solution is to automate the analytical method to counter for such bottleneck congestion in process development. Automation enhances high-throughput sample analysis yet retains consistency in the method of analysis.1, 2
Various downstream analyses of biopharmaceutical process-derived impurities (e.g., Host cell protein/HCP, DNA, or protein A) must be separated from the recombinant compound to ensure purity of the biopharmaceutical product.1
For instance, unpurified protein A could generate immunogenic effects and contribute to unintended effects when the product is applied to patients. Moreover, such impurities may also affect the efficacy of the drug product.
Enzyme-linked immunosorbent assay (ELISA) is a plate-based assay that is designed to detect and quantify various biologics such as antibodies or proteins. In this assay, an antigen is immobilized to a solid surface and then complexed with an antibody that is linked to an enzyme.3, 4, 5 Detection is accomplished by assessing the conjugated enzyme activity via incubation with a substrate to produce a measurable product. The most crucial element of the detection strategy is a highly specific antibody-antigen interaction.
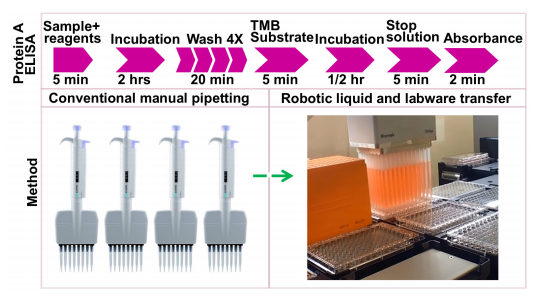
ELISAs are performed in 8-well polystyrene strips, 96 or 384-well polystyrene plates, where the antibodies bind to a target protein. The binding and immobilization of reagents makes ELISAs simple to design and perform. The reactants of the ELISA are immobilized to the microplate surface, which enables easy separation of bound from non-bound material, during the assay. Non-specifically bound materials are washed away, making ELISA assays a powerful tool for measuring specific analytes within a crude preparation.1 While ELISA methods are highly sensitive and specific, their accuracy and reproducibility are dependent on the analyst experience and expertise.1 The assay also contains multi-stage liquid transfer steps with varying volumes that can introduce pipetting errors to yield differential data across large data sets (Figure 1). ELISA automation can introduce consistency in the method of analysis, thus eliminating potential for human error, producing reliable results across samples. This application note describes an automated method by using the Beckman Coulter Biomek i7 hybrid workstation to perform protein A ELISA assay using a kit from Cygnus Technologies, Inc. Our results indicate successful automation of this assay, which can be directly applied to other ELISA protocols/kits.
Figure 1. Protein A ELISA workflow
Methods
Protein A ELISA
The ELISA manual experiments were performed following the recommended protocol of the protein A ELISA kit from Cygnus Technologies, Inc (Cat # F400Z). Standard curve analysis was performed using standards provided in the kit. A negative control sample containing sample diluent buffer was used. All experiments were performed in triplicates. Key equipment, reagents and resources used are listed in the material section. For automation, manual ELISA protocol was converted into Biomek methods using Biomek software.
Biomek methods
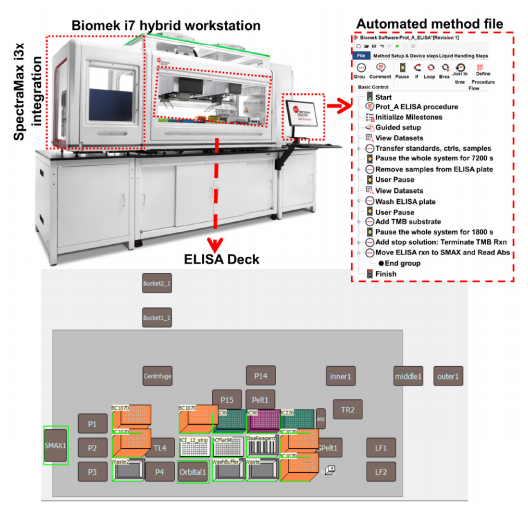
The automated experiments were performed by converting the pipette movement and device actions (Figure 1) to transfer liquid as recommended in the kit into Biomek methods using the Biomek software (Figure 2-right panel). Biomek i7 instruments have a high-density deck that can accommodate enough labware to use fresh tips for each transfer (Figure 2-bottom panel) yet retain the flexibility to integrate devices useful for the ELISA such as shakers, plate washers, and plate readers. The current experiments were performed on a Biomek i7 containing an integrated orbital plate shaker and a SpectraMax i3x plate reader (Molecular Devices). Shaking and incubations were performed using an on-deck orbital shaker. The 4x washing steps were also performed on deck using the Span-8 aspirate and dispense for the first three washes. In the final wash, select tip pipetting was used to aspirate and dispense the wash buffer, where the aspiration of the final wash buffer from the ELISA plate was performed by touching the edges of ELISA plate using the tips for complete removal of wash buffer and to automate the step that requires the use of absorbent pads in manual experiments. Analysis was performed using the standards provided in the kit for both the Biomek and manually performed experiments. Absorbance was measured using the SpectraMax i3x software v7.
Figure 2. Protein A automation on Biomek i7 hybrid automated liquid handler.
Results
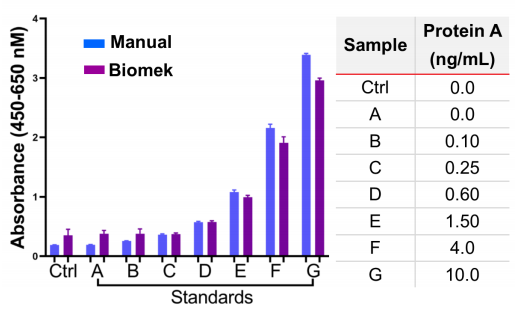
We compared the absorbance data acquired manually or by automated methods. There were no significant differences in the data obtained by either methods suggesting that both methods can be used interchangeably (Figure 3). A negative control was created by using sample diluent kit as suggested in the manual that had 0 ng/mL of protein A. For both Biomek and manually acquired datasets, the negative control sample had similar absorbance that was equal to that of standard A that also contains 0.0 ng/mL of protein A (Figure 3).
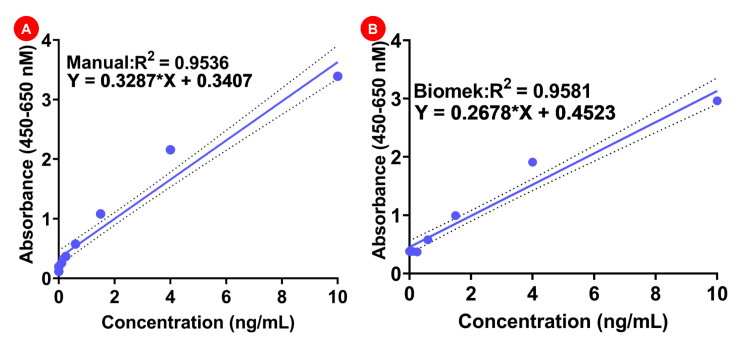
The validity of the data was analyzed using the linearity for the datasets generated using Biomek vs manual experiments. In the manual experiment the R2 value was 0.9536, which was strikingly similar to the R2 value from Biomek acquired datasets (0.9581) (Figure 4A-B). These findings suggest that automation of protein A can be successfully performed using Biomek i7 hybrid workstation, and that this approach does not interfere with the stability of the reagents and the reliability of the kit in determining the amount of protein A in a given sample.
Figure 3. Absorbance values for manual versus Biomek experiments.
Figure 4. Standard curve analysis for manual versus Biomek experiments. A) Manual experiment standard curve. B) Biomek experiment standard curve.
Summary
This application note demonstrated an automated method that can be used to measure the amount of protein A concentration in a sample. Data acquired manually versus via Biomek liquid handler were comparable without statistically significant differences. This suggests that the automated method can successfully replace the manual methods. Importantly, this approach can directly be applied to other ELISA protocols evaluating protein A analysis. Automation on Biomek systems can also be useful in settings with large-scale sample analysis needs. The flexibility to interchange between Span-8 and multi-channel pipetting enhances opportunities for high-throughput analysis. Labware integrations (e.g., plate shakers, plate washers, and plate readers) can contribute to throughput efficiency by decreasing interactions with the system to reduce user related error and increase walk-away time for the analyst. Moreover, the Biomek data acquisition and reporting tool (DART) is a software package designed to gather data and synthesize runtime information from Biomek log files capturing each manipulation of the sample during the Biomek method development. This eliminates time-consuming and error-prone “cut-and-paste” style management of sample data. DART seamlessly organizes data from Biomek liquid handlers, integrated lab equipment and benchtop devices into a single data source.
Bibliography
- G. . Rey and M. W. Wendeler, “Full automation and validation of a flexible ELISA platform for host cell protein and protein A impurity detection in biopharmaceuticals.,” Journal of Pharmaceutical and Biomedical Analysis, vol. 70, no. , pp. 580-586, 2012.
- J. L. Bock, “The new era of automated immunoassay.,” American Journal of Clinical Pathology, vol. 113, no. 5, pp. 628-646, 2000.
- C. K. Dixit, S. K. Vashist, B. D. MacCraith and R. . O’Kennedy, “Multisubstrate-compatible ELISA procedures for rapid and high-sensitivity immunoassays,” Nature Protocols, vol. 6, no. 4, pp. 439-445, 2011.
- S. Zhang, A. Garcia-D’Angeli, J. P. Brennan and Q. . Huo, “Predicting detection limits of enzyme-linked immunosorbent assay (ELISA) and bioanalytical techniques in general,” Analyst, vol. 139, no. 2, pp. 439-445, 2014
- R. M. Lequin, “Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA),” Clinical Chemistry, vol. 51, no. 12, pp. 2415-2418, 2005.
| Equipment | Manufacturer |
| Biomek i7 hybrid automated liquid handler | Beckman coulter Life Sciences |
| Spectramax i3x plate reader | Molecular Devices, Inc |
| Orbital shaker | Benchmark Scientific, Inc |
| Reagents | Manufacturer | Part Number |
| Protein A ELISA kit | Cygnus Technologies, Inc | F400Z |
| Diluent Buffer | I028 |
| Consumables | Manufacturer | Part Number |
| BC90 Pipette Tips | Beckman Coulter Life Sciences | B85884 |
| BC230 Pipette Tips | B85906 | |
| BC1070 Pipette Tips | B85945 |