High-throughput Miniaturization of Cytochrome P450 Time-dependent Inhibition Screening Using the Echo 525 Liquid Handler
Jing WangAbstract
Early assessment of the ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) properties of drug candidates has become a critical part of modern drug discovery. The frequent failure of compounds due to poor ADMET properties necessitates earlier profiling of ADMET characterizations. As a large number of compounds are screened for ADMET properties against various cytochrome P450 (CYP) enzymes on a regular basis, miniaturization and automation of ADMET assays have become one of the top priorities to manage the cost and labor. The time-dependent CYP inhibition assay is one of the most important ADMET assays which identifies drug candidates that may have potential for undesirable drug-drug interactions or sub-optimal pharmacokinetic properties. Here, we present a miniaturized, simplified and automated method using the Echo 525 Liquid Handler to evaluate compounds with time-dependent CYP inhibition potential with RapidFire/MS. The Echo 525 Liquid Handler can precisely and accurately transfer nanoliter droplets completely contact free to avoid any possible contamination. The rapid transfer of various aqueous reagents enables the evaluation of ADMET properties of a large number of compounds. In this application note, we discuss assay results from miniaturized time-dependent inhibition assays. The time-dependent inhibition for two of the most abundant CYP enzymes, CYP3A4 and 1A2, were evaluated. The results from the Echo Liquid Handler method demonstrate fast, reliable and accurate IC50 values.
Introduction
The Echo 500 series revolutionizes liquid transfer by using acoustic energy to eject fluids. The Echo 525 Liquid Handler is designed for rapid transfer of biochemical and genomics reagents for assay assembly. The Echo 525 transfers 25 nL droplets of most biochemical reagents. Microliter-scale volumes are rapidly transferred by repeating the 25 nL transfer hundreds of times per second. The Echo 525 system enables contamination-free reagent transfer to precisely and accurately build assays. Miniaturization with the Echo 525 Liquid Handler retains high assay performance, allowing quantitative results at higher densities. The Echo Liquid Handler can be used to transfer any volume to any well. These can be simple fluids (media for growing cells, buffer) or viscous solutions (lysis buffer, antibodies with glycerol, or transfection reagents).
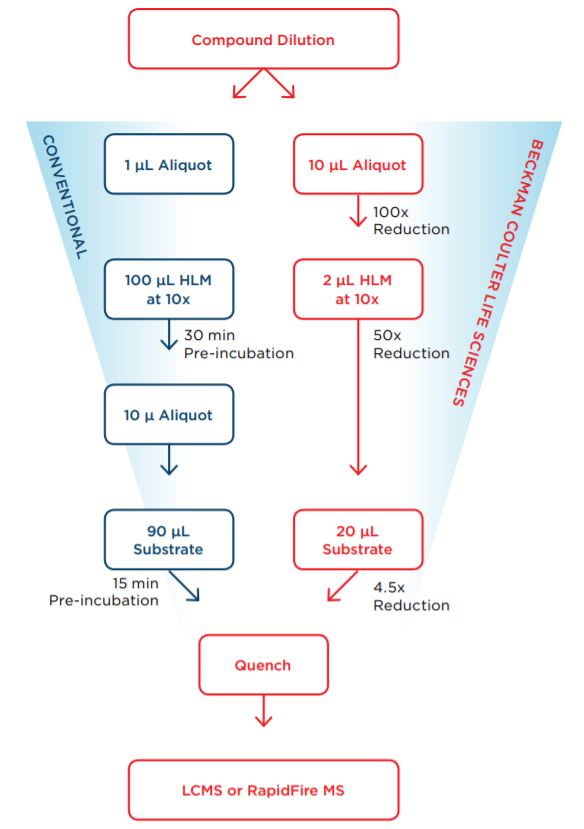
Accurate and precise transfer capabilities of the Echo 525 Liquid Handler reduce assay volumes and reagent cost, thus enabling cost-effective, time-dependent inhibition studies earlier in the drug discovery process. Figure 1 illustrates how the Echo 525 Liquid Handler enabled the reduction of reagent volume to conduct an effective experiment.
To demonstrate the capabilities of the Echo 525 in assay assembly and miniaturization, the reagents were transferred in 25 nL increments to a microplate prepared in advance with compounds. For this study, we used compounds of known time-dependent inhibition profiles against different cytochrome P450 (CYP450) isoforms. Mibefradil, Verapamil, and Mifepristone are time-dependent inhibitors against CYP3A4 and are utilized as posi-tive controls. Ketoconazole, a non-time-dependent inhibitor against CYP3A4, was used as a negative control. Similar experiments were undertaken with industry standard compounds to demonstrate time-dependent inhibition against CYP1A2.
Figure 1: Method comparison between conventional time-dependent CYP inhibition assay and Echo- enabled miniaturized time-dependent CYP inhibition assay
Time-Dependent CYP450 Inhibition Assay
The time-dependent CYP inhibition assay with the Echo 525 Liquid Handler was performed by incubating pooled Human Liver Microsomes (HLM) and test compounds in potassium phosphate buffer in the absence and presence of the cofactor NADPH. Upon completion of preincubation, substrate dissolved in potassium phosphate buffer containing NADPH was added. This reaction was further incubated for additional time in accordance with the CYP enzymes tested. At the end of incubation, acetonitrile was added to quench the reaction. Substrate metabolism was monitored by mass spectrometry. Changes in IC50 during incubation were assessed to determine if testing compounds had time-dependent inhibition. Compounds with greater than 3-fold changes in IC50 (ΔIC50) were generally considered to be time-dependent inhibitors.
Methods
Compounds were weighed out and dissolved in DMSO to make 50 mM stock solutions. Sixteen compound concentrations were generated in DMSO by direct dilution using the Echo® Dose-Response software application. 10 nL diluted compounds were transferred to two 384-well Greiner microplates with the Echo 555 Liquid Handler. 50 mM potassium phosphate buffer, pH 7.4 was made by combining potassium phosphate monobasic solu-tion and potassium phosphate dibasic solution. 1 mM NADPH was dissolved in the 50 mM potassium phosphate buffer. Both potassium phosphate buffer and NADPH buffer were kept in a 37°C water bath. HLM were diluted to 1.5 mg/mL in both potassium phosphate buffer and 1 mM NADPH buffer. 60 μL HLM in potassium phosphate buffer were dispensed into one column and 60 μL HLM in NADPH buffer were dispensed into another column of a 384-well Echo Qualified Polypropylene Plate. The Echo 525 Liquid Handler was used with the Echo® Plate Reformat software application to transfer 2 μL HLM in potassium phosphate buffer with or without 1 mM NADPH. The entire HLM transfer took approximately four minutes per 384-well microplate. The HLM and compound mixtures were then incubated in a 37°C water bath for 30 minutes. During the incubation, 5 μM 9-Amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate and 30 μM testosterone, all FDA-acceptable substrates, were made up in 1 mM NADPH buffer and kept in a 37°C water bath. At the end of the 30-minute incubation, 20 μL of 5 μM 9-Amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate were dispensed into one of the 384-well destination microplates. 20 μL of 30 μM testosterone were dispensed into another 384-well destination microplate. This reaction was further incubated for 15 minutes. At the end of the 15-minute incubation, 20 μL acetonitrile was added to quench the reaction. Bucetin was present in the quench solution as an internal standard. Quenched samples were cooled to 4°C, and centrifuged at 2500xg for 15 minutes. The assay plates were then used directly for sample injection by the RapidFire/MS system. Formation of 1-hydroxytacrine and 6β-hydroxytestosterone were monitored by mass spectroscopy.
Results
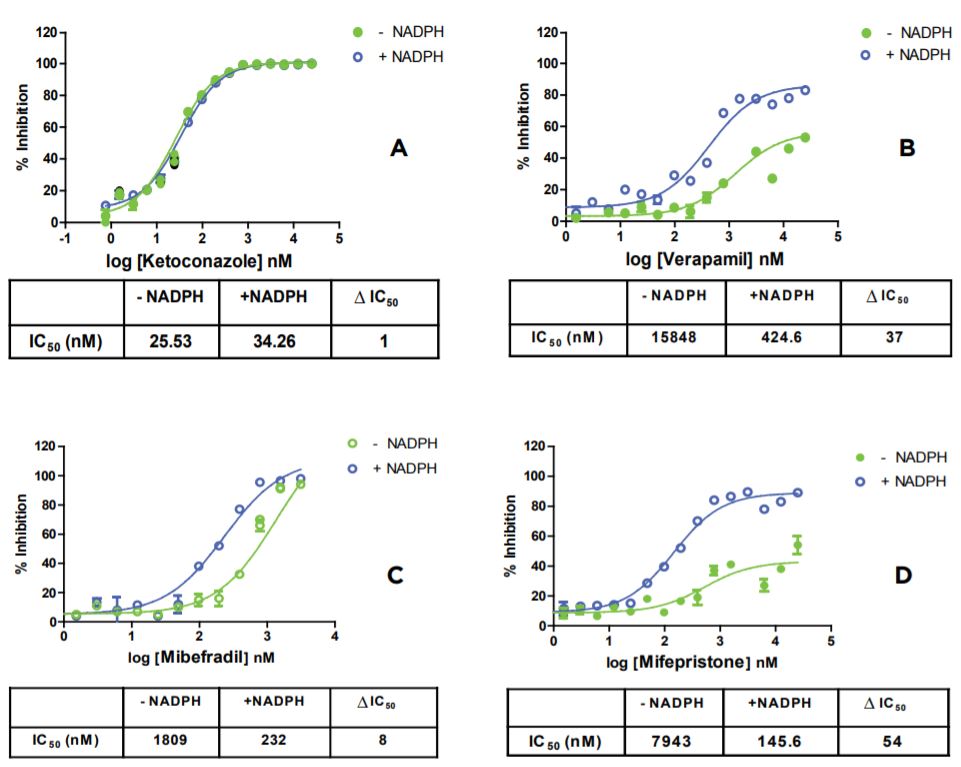
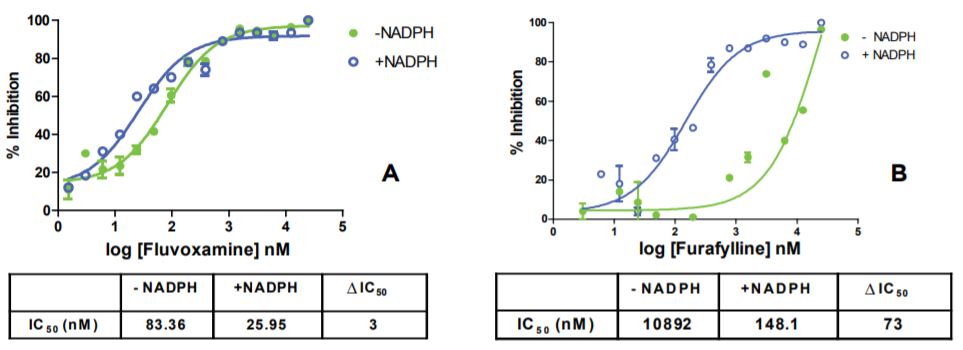
Miniaturized and simplified time-dependent CYP inhibition assays were developed with the Echo 525 Liquid Handler. Figure 2 demonstrates CYP3A4 inhibition profiles from four known inhibitors. Ketoconazole, a known and potent CYP3A4 inhibitor, showed no change in IC50 (ΔIC50 = 1) during incubation. Mibefradil, Verapamil, and Mifepristone, three known time-dependent inhibitors of CYP3A4, showed eight to fifty four-fold changes during incubation in the miniaturized time-dependent inhibition assay with the Echo 525 Liquid Handler. Data generated with the Echo 525 Liquid Handler showed consistent compound inhibition profiling when compared with published literature values. CYP1A2 inhibition profiles for two known inhibitors were also tested with the Echo 525 Liquid Handler. Figure 3 depicts Fluvoxamine, a potent CYP1A2 inhibitor, with a three-fold change in IC50 during incubation. Furafylline, a known time-dependent CYP1A2 inhibitor, showed a seventy three-fold change in IC50 during incubation in the miniaturized assay.
Figure 2: Time-dependent CYP3A4 inhibition profiling with four inhibitors (A-D).
Summary
Results from time-dependent CYP inhibition experiments can predict clinically relevant drug-drug interaction potential. Conventionally the assay is run with large reaction volumes to reduce DMSO concentration. Using the Echo 525 Liquid Handler and the Echo 555 Liquid Handler, compound volume can be reduced to 10 nL and microsomes volume to 2 μL. Results presented here demonstrate that miniaturized and simplified time-dependent CYP inhibition assays can be efficiently conducted with the Echo Liquid Handler. The Echo 525 Liquid Handler was used to demonstrate the capability to rapidly deliver low volumes of biochemical reagents in a tipless touchless manner. Superb data quality was indicated by precise and accurate curve fitting and IC50 results that were comparable with literature values. With the Labcyte Echo 525 Liquid Handler, scientists can now evaluate pharmacokinetic parameters in a reliable, rapid and cost-effective manner.
Materials
| Equipment | Manufacturer |
| Echo 525 Liquid Handler | Beckman Coulter Life Sciences |
| Echo 555 Liquid Handler | Beckman Coulter Life Sciences |
| RapidFire 200 MS System | Agilent Technologies |
| API 4000 System | AB Sciex |
| Reagents | Manufacturer | Part Number |
| InVitro CYP H-Class 10-Donor Pooled Human Liver Microsomes, Mixed Gender | Celsis, InVitro Technologies | #X008061 |
| 9-Amino-1,2,3,4-tetrahydroacridine hydrochloride hydrate | Sigma | #A3773 |
| Testosterone | Sigma | #T1500 |
| Ketoconazole | Sigma | #K1003 |
| Mibefradil dihydrochloride hydrate | Sigma | #M5441 |
| Mifepristone | Sigma | #M8046 |
| Verapamil hydrochloride | Sigma | #V4629 |
| Furafylline | Sigma | #F124 |
| Fluvoxamine maleate | Sigma | #F2802 |
| Bucetin | Sigma | #B4027 |
| Potassium phosphate monobasic solution | Sigma | #P8709 |
| Potassium phosphate dibasic solution | Sigma | #P8584 |
| β-Nicotinamide adenine dinucleotide phosphate, reduced tetra(cyclohexylammonium) salt | Sigma | #N5130 |
| Acetonitrile | Sigma | #271004 |
| Consumables | Manufacturer | Part Number |
| Echo Qualified 384-well Polypropylene Microplates | Beckman Coulter Life Sciences | #P-05525 |
| 384-well Microplates | Greiner Bio-One | #781280 |
Download PDF