Miniaturized Enzo Life Sciences HDAC1 Fluor de Lys Assays Using an Echo Liquid Handler Integrated in an Access Laboratory Workstation
Linda Orren, Bonnie Edwards, John Lesnick, Carl PetersAbstract
Epigenetic targets continue to provide alluring opportunities for drug discovery efforts in the pharmaceutical industry. Such pursuits typically involve the investigation of a number of epigenetic enzymes, and screens for inhibitors of these targets could contribute to the rapid consumption of compound libraries in high-throughput campaigns. The acoustic dispensing technology utilized by the Echo 555 Liquid Handler enables nanoliter compound volume dispensing and has become the gold standard for delivering compounds for drug discovery efforts. The small volume dispensing of Echo Liquid Handlers has broader applications in drug discovery. The screening effort described in this application note utilized the 2.5 nL drop size capability of the Echo 555 Liquid Handler to dispense compounds and the larger volume 25 nL drop size capability of an integrated Echo 525 to dispense aqueous epigenetic reagents in an automated screening assay using the Access Laboratory Workstation.
Introduction
Epigenetics continues to emerge as an important target class for drug discovery and cancer research. Histone Deacetylase 1 (HDAC1) is an epigenetic enzyme that regulates gene transcription by removing acetyl groups from lysine residues on histones and causing chromatin condensation. Human cancers of the gastric system, prostate, colon and breast have demonstrated increased HDAC1 expression. As research programs scale to evaluate many new targets related to epigenetic function, new tools and techniques are required to enable efficient and reproducible high-throughput epigenetic screening. Enzo Life Science’s Fluor de Lys assay technology is widely used for assessing the activity of epigenetic activity inhibitors. These assays are readily adaptable to high-throughput screening on the Access Workstation (Figure 1). In this study, the Echo liquid handling systems were used to miniaturize an HDAC1 Fluor de Lys assay to 3 μL total volume in 384-well microplates for a 10 plate automated screening run. Prior to assembling assays, 25 nL of epigenetic library compounds and control inhibitors were transferred with the Echo 555 Liquid Handler to generate assay-ready plates. The assay-ready plates were then transferred to an Access workstation with an integrated Echo 525 Liquid Handler for automated, high throughput assay assembly. On the Access workstation, the Fluor de Lys assay reagents were delivered from the Echo Qualified Reservoir using the Echo 525 Liquid Handler (Figure 2). Employment of acoustic energy transfer enabled by Echo liquid handlers to generate assay-ready plates and deliver Fluor de Lys reagents resulted in a robust, automated and miniaturized assay that accurately identified known HDAC1 inhibitors.
Figure 1: The Access Laboratory Workstation
Figure 2: Echo Qualified Reservoir
HDAC1 Fluor de Lys Fluorogenic Assays
The HDAC1 Fluorescent Activity Assay/Drug Discovery Kit is a complete assay system designed to measure the deacetylase activity of recombinant human HDAC1. The deacetylase activity is assessed by measuring the conversion of fluorophore-labeled peptide substrate.
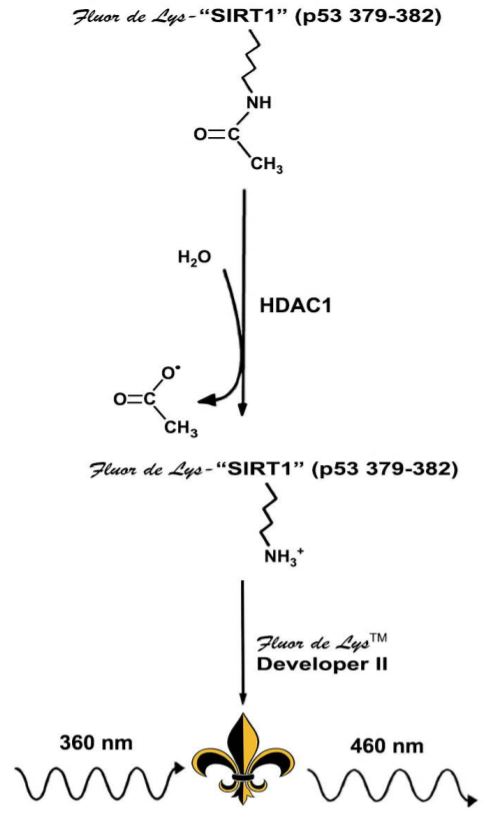
In the enzymatic reaction, the HDAC1 enzyme catalyzes the removal of the acetylated lysine side chain from the Fluor de Lys substrate. Deacetylation of the substrate sensitizes the substrate so that, in the detection step, treatment with the Fluor de Lys Developer produces a fluorophore(Figure 3).
Diagram courtesy of Enzo Life Sciences, Inc.
Figure 3: Enzo Life Science’s Fluor de Lys Assay Technology
Methods
HDAC1 Enzyme Titration: Experiment 1
The enzymatic activity of the HDAC1 enzyme was investigated in Enzo Life Science’s Fluor de Lys Fluorogenic assay.
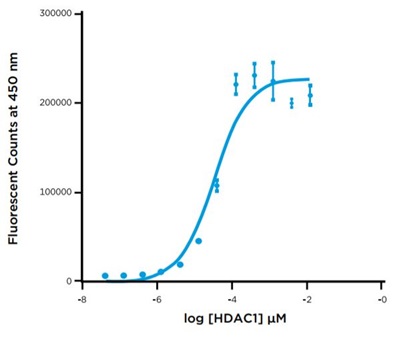
A 12-point, 1:3 serial titration of HDAC1 was prepared in the assay buffer provided in the Fluor de Lys kit. 1.25 μL of each dilution were transferred in triplicate along with 1.25 μL of 10 μM Fluor de Lys substrate from a 384-well Echo qualified polypropylene source plate to a 384-well assay plate using an Echo 525 liquid handler. The plate was then sealed and incubated at room temperature for 60 minutes. After incubation, 2.5 uL of the Trichostatin stop reagent /Developer II detection reagent cocktail was added from a 384-well Echo-qualified source plate to the assay plate using the Echo 525 liquid handler. The plate was re-sealed, and incubated for 60 minutes. After incubation, the plate was read on the PHERAstar FS and the Fluorescent signal was plotted versus log HDAC1 concentration (Figure 4).
Figure 4: HDAC1 Enzymatic Titration
Automated HDAC1 Screening Run: Experiment 2
The SCREEN-WELL Epigenetic Compound Library from Enzo Life Science contains many known inhibitors of HDAC1. To determine the ability of the HDAC1 Fluor de Lys assay to accurately identify inhibitory compounds, the library was tested in an automated assay run using an Echo 525 Liquid Handler integrated onto the Access workstation.
Prior to initializing the automation run, ten assay-ready plates were prepared using an Echo 555 liquid handler : seven plates containing the Trichlostatin A (TSA) and CUDC-907 inhibitory control titrations as well as control wells representing the total and background signal of the assay and three plates containing 6 copies per plate of each library compound in 25 µM as well as the aforementioned inhibitory titrations and assay controls.
| Total Volume | 5 μL | 2.4 μL |
| Compound / Buffer | 50 nL | 25 nL |
| Enzyme | 1.2 μL | 0.575 μL |
| Fluor de Lys substrate | 1.25 μL | 0.6 μL |
| Detection cocktail | 2.5 μL | 1.2 μL |
Table 1: Assay reagent volumes
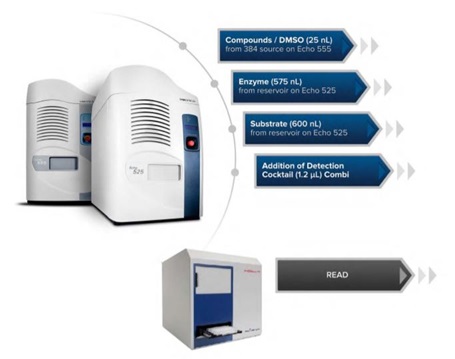
The assay ready plates were loaded onto the Access workstation. Using Tempo automation software, a schedule of tasks was executed to automate assembly of the assay: plates were delivered to the Echo system for addition of the enzymatic components of the assay (according to the volumes in Table 1) from an Echo qualified reservoir, then sealed, centrifuged and incubated at room temperature for one hour. The plates were then peeled and delivered to the Thermo fisher Multidrop Combi Reagent Dispenser for an addition of the TSA stop reagent /Developer II detection reagent cocktail, and then sealed, centrifuged and incubated for one hour at room temperature before being read on the BMG Pherastar FS (see Figure 5 for the assay workflow). Percent Inhibition for the control and library compound values were calculated using the following formula:((Total signal – sample signal)/(Total signal – background signal))*100.
For the control inhibitor titration, the percent inhibition values were plotted against the logarithm of the inhibitor concentration.
Figure 5: An HDAC1 Automated Workflow
Discussion
HDAC1 Titration: Experiment 1
HDAn HDAC1 enzyme titration was performed using the Echo system for precise delivery of all of the assay components to a 384-well plate to reach a total volume of 5 uL. The HDAC 1 titration demonstrates a broad dynamic range and provides the user information about the proper enzyme concentration to use for future experiments. Development experiments like these are very straightforward to perform on the Echo system as all of the reagents were delivered with acoustic energy, resulting in a reduction of both hands-on-time and reagent consumption. The resulting data indicated that 40 nM HDAC1 would provide sufficient assay signal for the screening run.
Automated HDAC1 Compound Screen: Experiment 2
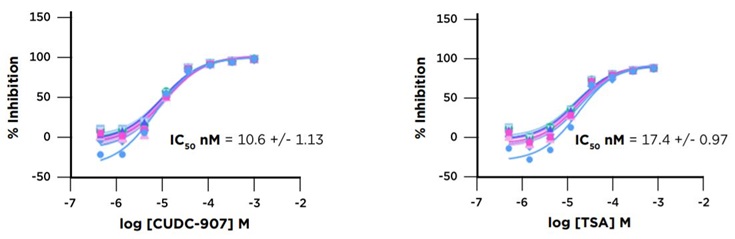
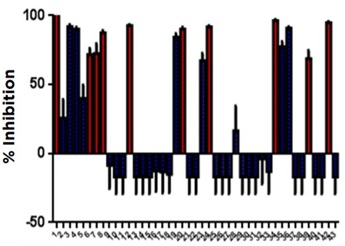
The ten plate HDAC1 screening run conducted on our Access Workstation with an integrated Echo 525 liquid handler produced high quality data and accurately identified HDAC1 inhibitors. The CUDC907 and TSA control showed very consistent inhibition over the course of the run (Figure 6). The screening run had a representative Z’ value of 0.72. Additionally, known HDAC1 inhibitors were identified by their strong percent inhibition values when tested at 25 µM. The consistency of the assay is also demonstrated by the small error bars in the percent inhibition determinations (Figure 7).
Figure 6: CUDC-907 and TSA Control Titrations
Figure 7: Percent Inhibition of SCREEN-WELL Library compounds
| 1. Trichostatin A | 16. Tranylcypromine hemisulfate | 31. BIX-01294 |
| 2. 2,4-Pyridinedicarboxylic Acid | 17. Valproic acid | 32. Butyrolactone 3 |
| 3. Garcinol | 18. Ex-527 | 33. CTPB |
| 4. Splitomicin | 19. Resveratrol | 34. Oxamflatin |
| 5. BML-210 | 20. M-344 | 35. Sirtinol |
| 6. Apicidin | 21. Nicotinamide | 36. Suramin•6Na |
| 7. Suberoyl bis-hydroxamic acid | 22. BML-266 | 37. BML-278 |
| 8. Scriptaid | 23. Piceatannol | 38. NCH-51 |
| 9. Nullscript | 24. Fluoro-SAHA | 39. CI-994 |
| 10. 5-Aza-2’-deoxycytidine | 25. Valproic acid hydroxamate | 40. NSC-3852 |
| 11. Zebularine | 26. AGK2 | 41. Aminoresveratrol sulfate |
| 12. SAHA | 27. Salermide | 42. BML-281 |
| 13. Isonicotinamide | 28. MC-1293 | 43. Triacetylresveratrol |
| 14. ITSA-1 | 29. Anacardic acid | |
| 15. Phenylbutyrate•Na | 30. B2 |
Summary
This workflow highlights the power and flexibility of acoustic liquid handling in the lab. The Echo 555 Liquid Handler was employed to rapidly and accurately dispense small volumes of compounds and controls to the assay-ready plates. The Access workstation with an integrated Echo 525 Liquid Handler completely automated this 10 plate screening run. The Echo Qualified Reservoir was used to deliver common reagents like HDAC1 enzyme and Fluor de Lys substrate to the assay plates accurately and with little volume wasted due to minimal dead volume required.
The employment of acoustic dispensing in this miniaturized assay run resulted in the accurate and efficient identification of known HDAC1 inhibitors with a cost and reagent consumption savings of over 80%.
Materials
Access Workstation Components
| Component | Manufacturer |
| Access Workstation | Beckman Coulter Life Sciences |
| Echo 525 Liquid Handler | Beckman Coulter Life Sciences |
| Thermofisher Scientific Multidrop Combi Reagent Dispenser | Thermofisher |
| Agilent Technologies Microplate Centrifuge | Agilent |
| Agilent Technologies PlateLoc Thermal Microplate Sealer | Agilent |
| Brooks Life Sciences XPeel Microplate Peeler | Brooks |
Assay Reagents
| Assay | Manufacture | Part Number |
| Human recombinant HDAC1 | Enzo | BML-SE465-0050 |
| FLUOR DE LYS HDAC1 fluorometric drug discovery assay kit | Enzo | BML-AK511-0001 |
| Epigenetic SCREEN-WELL compound library | Enzo | BML-2836-0100 |
| 384-well Echo qualified polypropylene source plate | Beckman Coulter Life Sciences | 001-14555 |
| Echo Qualified Reservoir | Beckman Coulter Life Sciences | 001-11101 |
| 384-well assay plate | Greiner | 784075 |
| Trichostatin A | Selleckchem | S1045 |
| CUDC-907 | Selleckchem | S2759 |