Innovation in CD4 Testing
HIV Diagnosis Becomes up to Four Times Faster Thanks to This Invention
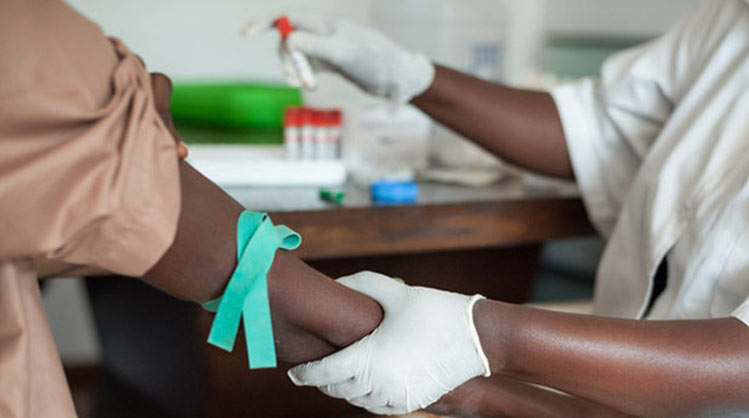
HIV/AIDS Africa’s poorest and most vulnerable can benefit from simple HIV test which monitors disease progression, enabling governments to deliver comprehensive treatment programs.
 Professor Debbie Glencross
Professor Debbie Glencross
Director and Principle Pathologist in the Flow Cytometry unit of the Department of Harmatology at the Charlotte Maxeke Johannesburg Academic Hospital
Improving diagnostics in rural communities
Prof Debbie Glencross is Director and Principle Pathologist in the Flow Cytometry unit of the Department of Hematology at the Charlotte Maxeke Johannesburg Academic Hospital, South Africa. Her breakthrough PLG (Pan-Leukocyte Gating) method is easy to use and requires no specialist training, unlike conventional techniques for CD4 monitoring.
“By making the testing process much simpler, both money and time can be saved.”
Effective HIV treatment relies on clinicians having an accurate CD4 count for a patient. However, in many parts of rural Africa, the equipment and infrastructure simply isn’t available to test patients, get their blood samples to a lab, and then report the results.
As Glencross explained: “Our objective is to empower smaller community laboratories so that they can extend the availability of PLG to meet demand, while still meeting the testing standards required by the National Health Laboratory Service (NHLS).”
Speeding up CD4 cell counting
When counting CD4 cells, large labs differentiate between the types of white blood cells, count them and then work out the number of CD4 cells in each millionth of a liter of blood. While accurate, this method can be laborious.
“A compact laboratory instrument is now available that offers high quality, automated CD4 counts in the near-patient setting.”
In contrast, rather than going through the time-consuming and costly process of isolating individual antibodies, Glencross’s approach uses a mathematical equation. She realized that using the white cell count as a stable reference point would eliminate the need for additional quality control steps, while still maintaining standards.
Working in partnership for maximum benefit
With the help of Beckman Coulter Life Sciences the Glencross’s PLG test was developed to use in conjunction the AQUIOS CL Flow Cytometer a more compact flow cytometry analyzer.
Together the two technologies form the AQUIOS PLG test, a simple cytometry flow test which uses a two-color, pre-optimized reagent and provides both CD4 percentages and absolute counts. Consequently, the test enables rural healthcare workers with limited training to carry out diagnostics in community-based laboratories.
Reporting time reduced by up to 400 per cent i
Resource-poor communities not only save time, but also much needed healthcare costs. It was reported at a recent conference of the African Society of Laboratory Medicine that the time it took to report results when using the new test could be reduced by as much as 400% (with the median time down from 12-38 hours to just 4-13 hours)ii.
“The rapid turnaround time and ease of use enables doctors and patients to get results in well under an hour, so that timely treatment decisions can be made.”
Glencross concluded: “The cost of our HIV/AIDS burden makes it essential that we find new and more cost effective ways of providing CD4 monitoring. The PLG test simplifies flow cytometry so that it can be carried out in these smaller labs nearer the patient. This reduces the turnaround time so that people can have their results more quickly- but without increasing costs.”
Timely treatment
Previously, blood samples had to be transported for several days to the larger flow cytometry centers. The combination of the simplified test and a powerful but compact analyzer changes this.
Dr. Jeannine T. Holden, Beckman Coulter’s Director of Scientific Affairs, pointed out: “A compact laboratory instrument is now available that offers high quality, automated CD4 counts in the near-patient setting. The rapid turnaround time and ease of use enables doctors and patients to get results in well under an hour, so that timely treatment decisions can be made.”
Fill the form to download comparison of PLG Assays

Innovation In CD4 Testing
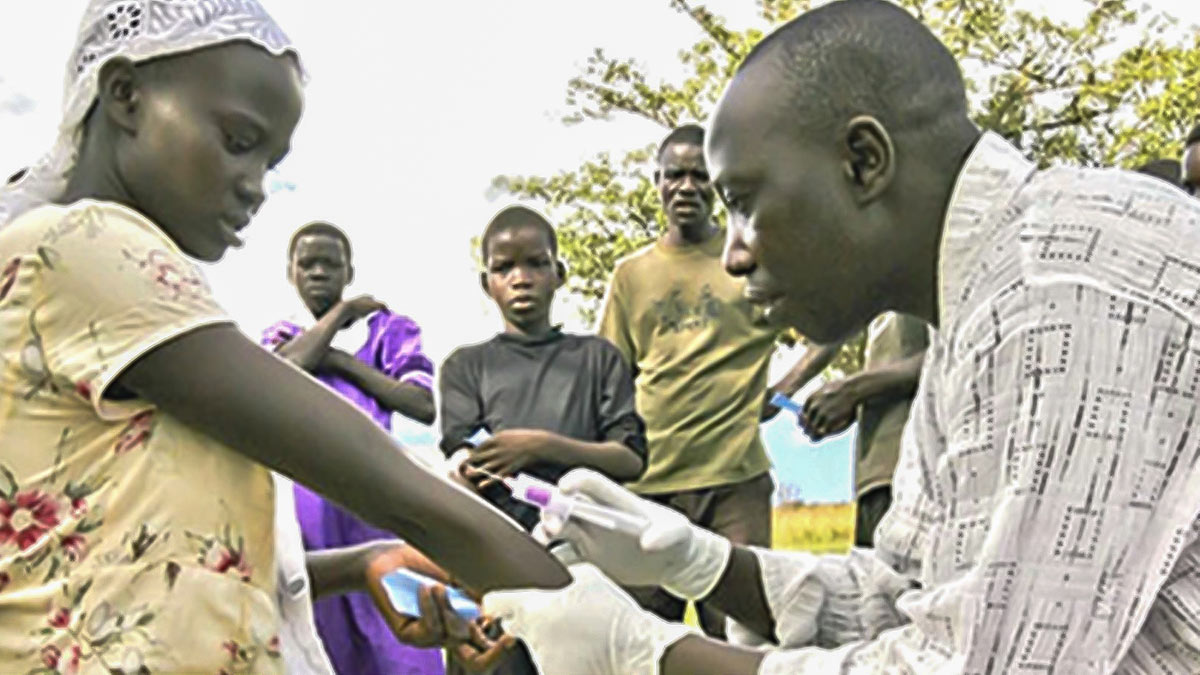
HIV Advanced Disease Management
More than a third of people starting ART have advanced HIV disease, and an increasing number of patients develop an advanced HIV after disengaging from care.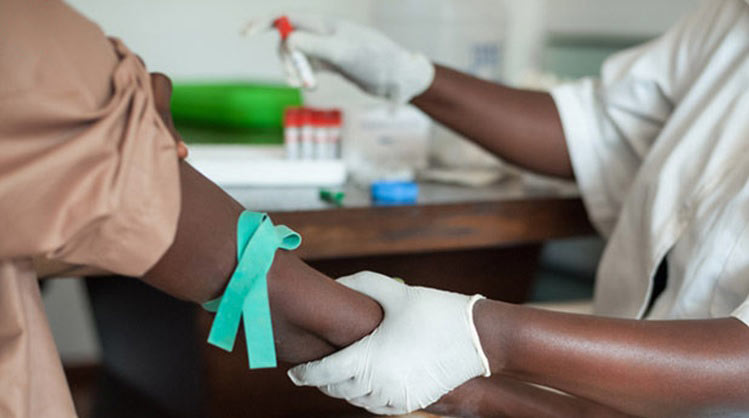
HIV Diagnosis Becomes up to Four Times Faster Thanks to This Invention
HIV/AIDS Africa’s most vulnerable can benefit from simple test which monitors disease progression, enabling the comprehensive treatment programs.
AQUIOS CL Flow Cytometer Accepted by WHO Prequalification of In Vitro Diagnostics Programme
The AQUIOS CL flow cytometer has been accepted by the World Health Organization (WHO) Prequalification of In Vitro Diagnostics Program.
Case Study: London Health Sciences Centre (LHSC)
AQUIOS CL provides faster times to first result, faster turnaround times and faster total elapsed times, with fewer total steps and less hands-on time.References
- "HIV diagnosis becomes up to four times faster thanks to this invention": Cassim N, Coetzee LM, Glencross DK (2014, 2015) Assessing the impact of implementing a Community CD4 Laboratory in a rural health district in South Africa. African Society of Laboratory Medicine: 1st International Conference. Cape Town and NHLS PathRED, Johannesburg.
- Coetzee LM, Cassim N, Glencross DK. Implementation of a new 'community' laboratory CD4 service in a rural health district in South Africa extends laboratory services and substantially improves local reporting turnaround time. S Afr Med J. 2015;106(1):82-7.
- 2016 Global Summary, WHO http://www.who.int/hiv/data/en/
- https://labtestsonline.org/conditions/hiv-infection-and-aids
- https://labtestsonline.org/tests/cd4-count

