London Health Sciences Centre Workflow Comparison Study with the AQUIOS CL Flow Cytometer
Laboratory Profile
- London Health Sciences Centre (LHSC), Flow Cytometry Laboratory, London, Ontario, Canada
- Operates Monday to Friday from 8 a.m. to 5 p.m. with an on call service for weekends and holidays
- Employs 5 medical technologists, 1 technical and medical supervisor, and 2 PhD research scientists
- Performs 6,000 tests / year
The flow cytometry laboratory at Victoria Hospital is situated within a tertiary care academic institution, London Health Sciences Centre. This lab serves more than 1,000,000 residents within London and the greater South Western Ontario region.
The flow cytometry laboratory in London Health Sciences Centre performs over 6,000 clinical flow cytometry tests per year in addition to numerous research projects.
The lab is staffed by five full-time, registered medical technologists, in addition to a technical and medical supervisor. Two PhD students also work in the laboratory in translational research and development projects.
Overview of Traditional Flow Cytometry Workflow
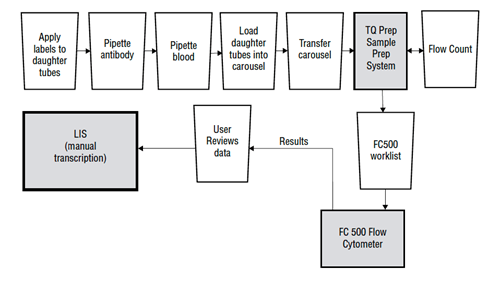
Many traditional flow cytometry systems, such as the current Beckman Coulter FC500 with TQ Prep, require either manual or semi-automated preparation of samples, manual creation of worklists, manual data review, and manual tabulation of numerical data. This workflow can result in longer run times, more hands-on time, and require more experienced operators. See Figure 1 for the flow cytometry workflow at London Health Sciences Centre.
Figure 1. London Health Sciences Centre (LHSC) Flow Cytometry Workflow Example
Overview of the AQUIOS CL Flow Cytometer Workflow
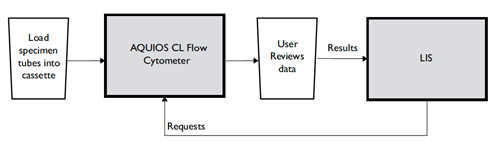
The AQUIOS CL system is a fully-automated system that performs all of the preparatory steps leading to a test result. See Figure 2 for the AQUIOS CL Flow Cytometer workflow.
Figure 2. AQUIOS CL Flow Cytometer Workflow
Comparison Protocol
The purpose of this case study is to evaluate the AQUIOS CL Flow Cytometer design and its resultant workflow through direct comparison with the Beckman Coulter FC500 and TQ Prep system. This case study evaluates the following workflow parameters:
-
Total elapsed time from startup to shut down – The time duration that begins with the first step in starting up the instrument(s) and ends at the last moment of shutting down the instrument(s) when all instrument and software activity has ceased.
-
Time to first result from startup – For the AQUIOS CL system and alternate systems, the time duration that begins the moment the system is turned on and ends with the first test result for the same sample displayed on the instrument computer screen, for a batch of 10, 25, or 50 samples.
-
Turnaround time – For alternate systems, the time duration begins with the first sample preparation step and ends with completed test results for a batch of 10, 25, or 50 samples, as demonstrated by the last sample test result displayed on the instrument computer screen. For the AQUIOS CL system, time duration begins with the first cassette placed in the autoloader and ends with the completed test result for the last sample being displayed on the instrument computer screen, for a batch of 10, 25, or 50 samples. The calculation is total time divided by the number of samples. The time does not include QC.
-
Number of procedural steps – The number of instructions or actions required to accomplish the task. This includes all the distinct steps in the process of generating a test result.
To avoid individual interpretation of the meaning of a “step,” the protocol adheres to the steps as defined by the manufacturer in the applicable Instructions for Use manual. For example, if the Instructions for Use manual states to ‘pipette 100 μL of blood,’ this is one step. Sub-steps such as ‘insert a pipette tip on the pipette, push down the plunger on the pipette, insert the pipette in the blood sample’ are not considered steps unless the manufacturer specifies them as such.
It is important to note that the AQUIOS CL Flow Cytometer and alternate systems do not employ identical procedural steps. Therefore, the “steps” are categorized by function even if it is not identical system-to-system.
It should also be noted that, for the AQUIOS CL Flow Cytometer the procedural steps for Tetra-1 and Tetra-2+ tests are identical.
-
Operator hands-on time – The time required by the user to physically perform the steps listed in the provided test procedure.
Note: The workflow parameters measured by this case study does not affect safety or effectiveness
Study Controls
To ensure standardization between both comparison platforms, the following controls were used:
- The same samples are run on both systems.
- Both systems utilize the manufacturer’s 4-color, lymphocyte subset panels using CYTO STAT tetraCHROME CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5 for the FC500 Flow Cytometer and AQUIOS Tetra-1 for the AQUIOS CL Flow Cytometer
- Beckman Coulter’s IMMUNO-TROL and IMMUNO-TROL Low Controls are run on the FC500 Flow Cytometer and AQUIOS IMMUNO TROL and AQUIOS IMMUNO-TROL Low controls are run on the AQUIOS CL Flow Cytometer for each of the sample numbers studied.
- The AQUIOS CL Flow Cytometer and the FC500/TQ Prep systems are run per the Instructions for Use manuals for each system.
- In all instances, the AQUIOS CL Flow Cytometer utilizes the autoloader system.
- In all instances, one individual performed all steps on both systems. The individual was not permitted to multitask between the two systems.
- Control test requests were created prior to initiating the study following the system Instructions for Use manual.
Comparing the FC500/TQ Prep System to the AQUIOS CL Flow Cytometer
Total Elapsed Time from Startup to Shutdown
This test case consisted of 10, 25, and 50 sample scenarios. The AQUIOS CL autoloader system, running AQUIOS Tetra-1 was compared directly to the FC500 and TQ Prep systems, running CYTO-STAT tetraCHROME CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5. Note that the 50 sample scenario required 3 batches on the FC500 and TQ Prep systems.
Refer to Table 1 for a tabular description of the total elapsed time results.
Table 1. Hands-On/Hands-Off Time and Total Elapsed Time
Note: The time format is displayed as (h:mm:ss). For example, 1:01:01 equals one hour, one minute, and one second.
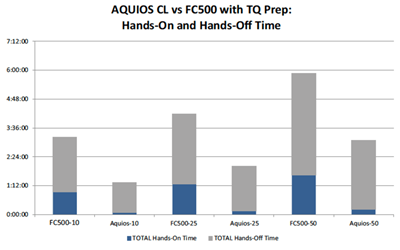
Refer to Figure 3 for a graphic depiction of the total elapsed time results. Note that combining the Total Hands-On Time (blue bar) with the Total Hands-Off Time (gray bar), depicts total elapsed time.
Figure 3. Hands-On/Hands-Off Time and Total Elapsed Time
Legend Key: The number after the cytometer name represents the number of samples run.
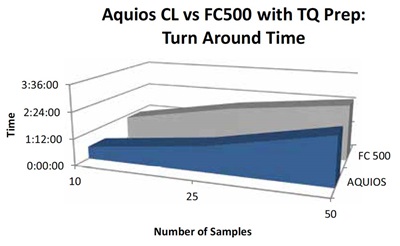
In the 10 sample scenario, the AQUIOS CL Flow Cytometer is 2.4X faster than the FC500 and TQ Prep system. In the 25 sample scenario, the AQUIOS CL Flow Cytometer is 2.1X faster than the FC500 and TQ Prep system. In the 50 sample scenario, the AQUIOS CL Flow Cytometer is 1.9X faster than the FC500 and TQ Prep system. Assuming 50 samples per workday over the period of one year, the AQUIOS CL Flow Cytometer saves a lab hundreds of hours of valuable time each year. With such a quick turn-around time, the AQUIOS CL Flow Cytometer is capable of more work in less time and has the capacity to do the same work in less than half the time of a traditional Flow Cytometer combination like the FC500 and TQ Prep system.
Amount of Hands-On Time Required
In Figure 3, the Total Hands-On Time is depicted by the blue bar and the total Hands-Off Time is depicted by the gray bar.
In all test case scenarios, the AQUIOS CL Flow Cytometer required significantly less hands-on time than that of the FC500/TQ Prep system combination. This can be attributed to the integrated sample preparation features in the AQUIOS CL Flow Cytometer. The all-in-one approach of the AQUIOS CL Flow Cytometer is intended to assist in time savings and to help increase the workflow efficiency.
Number of Procedural Steps Required
This test case consisted of 10, 25, and 50 sample scenarios. The AQUIOS CL autoloader, running AQUIOS Tetra-1 was compared directly to the FC500 and TQ Prep systems running CYTO STAT tetraCHROME CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5. See Table 2 for a breakdown of the results. The Instructions for Use manuals were used by the investigator to determine the number of steps for each category.
Note: LHSC reported number of steps by multiplying by the number of specimens. The table below shows the number of steps for each specimen.
Table 2. Number of Procedural Steps
Steps related to sample preparation, quality control and running samples contributed to the distinct difference in the total number of steps between both systems. Regardless of the number of samples in each test case, the AQUIOS CL system consistently reported steps in significantly lower quantity than the FC500/TQ Prep system setup. In addition, the number of steps remained stable across all sample scenarios due to the fully automated design of the AQUIOS CL Flow Cytometer. The reduced number of steps on the AQUIOS CL Flow Cytometer offers a probable correlation to the reduced hands-on time reported in the section above.
Turnaround Time
This test case consisted of 10, 25, and 50 sample scenarios. The AQUIOS CL autoloader, running AQUIOS Tetra-1 was compared directly to the FC500 and TQ Prep systems running CYTO STAT tetraCHROME CD3/CD8/CD45/CD4
Three results were measured: time to first result from startup, turn-around time, and total elapsed time. Refer to Table 3 for the results.
Table 3. Turnaround Time Results
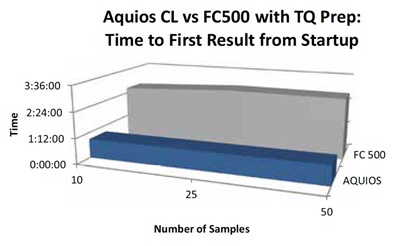
Figure 4 depicts time to first result from startup for both the AQUIOS CL system and the FC500/TQ Prep system. The time duration in this instance begins when the system is turned on and ends with the first test result displayed on the workstation. The AQUIOS CL system delivered the first result approximately three times faster than the FC500/TQ Prep system for all three sample scenarios.
Figure 4. Time to First Result from Startup
Figure 5 depicts turn-around time for both the AQUIOS CL system and the FC500/TQ Prep system. The time duration in this instance began with the first sample preparation step (FC500/TQ Prep system) or the first cassette being placed on the system (AQUIOS CL system) and ended with the last test result displayed on the workstation. The AQUIOS CL system delivered faster turn-around times in all three sample scenarios. The 10 sample scenario turn around time on the AQUIOS CL system was 1.9 times faster than that of the FC500/TQ Prep system. The 25 sample scenario on the AQUIOS CL system was 1.7 times faster, and the 50 sample scenario on the AQUIOS CL system was 1.2 times faster than that of the FC500/TQ Prep system.
Figure 5. Turn-Around Time
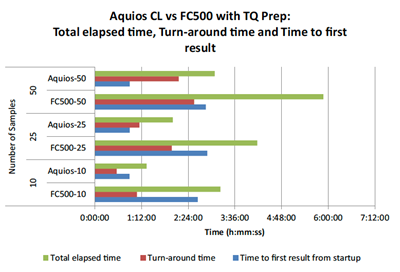
Figure 6 depicts total elapsed time, turn-around time, and time to first result for both the AQUIOS CL system and the FC500/TQ Prep system. In all three instances including total elapsed time, turn-around time, and time to first result from startup, the AQUIOS CL Flow Cytometer consistently delivered faster results in this case study.
Figure 6. Total Elapsed Time, Turn-Around Time, and Time to First Result
Conclusions
The study indicates that the AQUIOS CL Flow Cytometer performs well compared to a traditional flow cytometry system by providing faster times to first result from startup, faster turnaround times and faster total elapsed times than the comparator, with fewer total steps and less hands-on time, when processing 10, 25 and 50 samples in the given test case.
There are two important factors to note regarding this study. The first factor to note is that the time savings exhibited in this study are conservative estimates. A principal advantage of the AQUIOS CL system, freedom from batching, is not measured as a test case. Most current flow cytometry laboratories including FC500/TQ Prep system users have workflows that involve waiting until a critical mass of samples arrive in the lab before starting sample preparations (batching). An AQUIOS CL system user, however, can start running as soon as the first sample arrives. This amounts to improved workflow results in greater time savings than the study indicates. Additionally, the lab can load new samples at any time during the hours of operation with the AQUIOS CL Flow Cytometer. Due to the workflow, traditional flow cytometers require samples to be held until the next morning at a designated time before closing. The second factor to note is that with most traditional flow cytometers, the operator tends to stay at or near the instrument to monitor progress. If there is not a lot of time in between steps, for example five minutes or ten minutes, the user tends to remain close to the instrument and monitor the data acquisition in real- time rather than walk away to accomplish another task. The time that a user is monitoring the data acquisition is not accounted for in this study. The Load & Go nature of the AQUIOS CL system actually accounts for greater time savings and work efficiency than actually indicated by this study since a user can accomplish more in less time.
Aquios CL is a Class 1 laser product.
Fill the form to download Workflow Comparison Study with AQUIOS CL Flow Cytometer

Innovation In CD4 Testing
HIV Advanced Disease Management
More than a third of people starting ART have advanced HIV disease, and an increasing number of patients develop an advanced HIV after disengaging from care.
HIV Diagnosis Becomes up to Four Times Faster Thanks to This Invention
HIV/AIDS Africa’s most vulnerable can benefit from simple test which monitors disease progression, enabling the comprehensive treatment programs.
AQUIOS CL Flow Cytometer Accepted by WHO Prequalification of In Vitro Diagnostics Programme
The AQUIOS CL flow cytometer has been accepted by the World Health Organization (WHO) Prequalification of In Vitro Diagnostics Program.